Removal of cadmium ions from aqueous solution via micellar-enhanced ultrafiltration
HUANG Jin-hui(黄瑾辉), ZENG Guang-ming(曾光明),XU Ke(许 柯), FANG Yao-yao(方瑶瑶)
(Department of Environmental Science and Engineering, Hunan University, Changsha 410082, China)
Abstract: To separate cadmium ions from aqueous solution efficiently, micellar-enhanced ultrafiltration(MEUF) of hollow ultrafiltration membrane was used, with sodium dodecyl benzene sulfonate (SDBS) and sodium dodecyl sulfate (SDS) as surfactants. The important parameters affecting the rejection of cadmium, the permeate flux and the secondary resistance were investigated, including surfactant species, surfactant concentration, operating time, trans-membrane pressure, the addition of electrolyte and solution pH. The results show that the rejection rate of cadmium reaches 97.8%. Trans-membrane pressure and the addition of electrolyte (NaCl) are less influential while surfactant species, surfactant concentration and pH value are important for micellar-enhanced ultrafiltration. The optimum concentration of surfactant is the critical micelle concentration, and SDBS is better than SDS. Micellar-enhanced ultrafiltration with SDBS can separate cadmium ions from aqueous solution efficiently.
Key words: micellar-enhanced ultrafiltration; cadmium ions; surfactants; micelles CLC number: TQ028.8
Document code: A
1 INTRODUCTION
Wastewater containing cadmium may be classified by different sources, including cadmium mine smelting process, cadmium compound manufacturing industries, nickel-cadmium cell manufacturing process and cadmium-plating industries. Cadmium could be enriched in human body and cause serious diseases or bring chronic illness through food chain, which is among the pollutants of No.1 group prescribed in integrated wastewater discharge standard in China. At present, the processes frequently adopted for removing cadmium from wastewater are concerned with chemical precipitation, chlorinated lime oxidation, adsorption, ion-exchange and membrane separation. However, each process has its own limits, such as low separating efficiency, difficulty to dispose the sediment, impossiblity to recycle cadmium, expensive cost. For many years, scholars have been working hard to seek for new technology to separate cadmium from aqueous solution.
Micellar-enhanced ultrafiltration(MEUF) was originated in 1968 when Michaels proposed to use polymer or surfactant modified ultrafiltration. In 1979, Leung first removed trace metal ion by using MEUF, and since then this technology has been studied. The method was easy to operate, whats more, it is indicated that in this experiment the rejection rates of Pb2+, Zn2+, Ni2+, Cu2+ and Ca2+ were higher than 99%, and the metal ions were also easily recovered from retentate solution through MEUF. But restricted by the characteristic of the material of membrane, the frame of membrane module and expensive cost, MEUF was still at the experimental stage.
Since 1980s, membrane module has been well developed. More and more membrane antipollution materials have been developed and the cost of membrane was reduced. All these signs suggest that MEUF is more and more promising. In recent studies, almost all the metal ions can be separated via MEUF method, including Cd2+[5], Ni2+[6, 7], Co2+, Cs+, Sr2+, Cr3+, Mn2+[8], Pb2+[9], CrO3-4[10], Zn2+, Cu2+[11], AuCl-4[12] and Fe(CN)3-6[13]. The single ionic surfactants turn to mixed surfactants. For example, Cu2+[14] was rejected by mixed SDS/Triton X-100 surfactants, and Cr3+[15] was rejected by mixed SDS/NPE surfactants. The material of ultrafiltration membrane involves hydrophilic polyamide and hydrophobic polysulfones. There are various membrane modules including plate ultrafiltration membrane, hollow fiber ultrafiltration membrane and spiral-type pipe ultrafiltration membrane.
Researches into the application of MEUF to separate cadmium from aqueous solution at present focus on surfactant species, surfactant concentration, and trans-membrane pressure. But pH and electrolyte (NaCl) concentration in wastewater are different, so they cannot be ignored.
Cadmium ions were separated from aqueous solution via micellar-enhanced ultrafiltration by using sodium dodecyl benzene sulfonate (SDBS) and sodium dodecyl sulfate (SDS) as surfactants in our research. The effects of important parameters are investigated, including surfactant species, surfactant concentration, trans-membrane pressure, operating time, the addition of electrolyte (NaCl) and pH value.
2 MECHANISMS OF MICELLAR-ENHANCED ULTRAFILTRATION
The removal of cadmium ions via micellar-enhanced ultrafiltration includes three procedures. The first procedure is micelle formation, namely surfactants dissolved in water. While surfactant concentration exceeds critical micelle concentration (CMC), SDBS/SDS monomers tend to self-aggregate to form spheriform micelles. These micelles are usually composed of 50-150 monomers, whose mass is quite large. The diameter of micelle is 0.005-0.010μm. The CMC of SDBS and SDS are 1.2×10-3mol/L(0.42g/L) and 8×10-3mol/L(2.30g/L), respectively. The second procedure is the adsorption of micelles. SDBS/SDS is cation surfactant, whose micelles are of negative charges on surface. Cadmium ions in the solution are adsorbed by micelles via electrostatic attraction. The third procedure is micellar ultrafiltration. When SDBS/SDS micelles with cadmium ions are filtrated by membrane, micelles with cadmium ions are rejected because of their large particle diameter, while water, trace SDBS/SDS and cadmium ions permeate. Therefore, cadmium ions are separated from aqueous solution. The principle is shown in Fig.1.

Fig.1 Principle of micellar-enhanced ultrafiltration
3 EXPERIMENTAL
3.1 Materials
SDBS used in the research was produced by Shanghai Yingpeng Additive Chemical Plant Limited Co., China. Its molecular formula is C18H29-NaSO3, with purity of 85%. SDS was produced by Tianjin Miou Chemic Reagent Empolder Center. Its molecular formula is C12H25NaSO4, with purity of 99%. Cadmium ions were confected by cadmium nitrate. Cadmium nitrate is produced by Shanghai Tinxin Chemical Reagent Plant. Its molecular formula is Cd(NO3)2·4H2O, with purity of 99%.
3.2 Experimental procedure
SDBS/SDS was added into cadmium ions aqueous solution. After being full mixed, the solution was fed into membrane module for linear continuous ultrafiltration by wriggle pump. The procedure is shown in Fig.2.
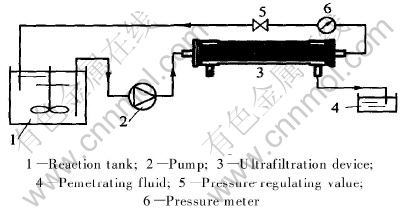
Fig.2 Schematic of micellar-enhanced ultrafiltration process
3.3 Membrane module and characteristic of membrane
3.3.1 Membrane module
Ultrafiltration membrane was hollow core fiber ultrafiltration membrane produced by Tianjin Motianmo Co., China. Its type is UEOS503. The characteristic of membrane is shown in Table 1.
3.3.2 Permeate flux[16]
The water permeate flux and solution permeate flux of ultrafiltration membrane were measured in different trans-membrane pressures.
Ji=Qi/A(1)
where Ji is the permeate flux, m3/(m2·s); Qi is the feed flux, m3/s; A is the area of membrane, m2.
Table 1 Membrane characteristics

3.3.3 Rejection rate
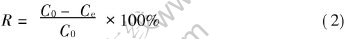
where C0 is the concentration of Cd2+ in the feeding solution, mg/L; Ce is the concentration of Cd2+ in the percolate, mg/L.
3.3.4 Resistance
The resistance of ultrafiltration membrane in micellar-enhanced ultrafiltration includes the resistance of membrane and the secondary resistance which was caused by the pollution of membrane.

where Rm is the resistance of membrane, m-1; Rf is the secondary resistance, m-1; Δp is the trans-membrane pressure, Pa; μw is the viscosity co-efficient of water, Pa·s; μs is the viscosity coefficient of solution, Pa·s; Jw is the permeate flux of water, m3/(m2·s); Js is the permeate flux of solution, m3/(m2·s).
In this study the permeate flux of water and the permeate flux of solution were determined under the same trans-membrane pressure. The permeate flux of water was 5.29×10-6m3/(m2·s) when the trans-membrane pressure equaled 0.07MPa.
3.4 Analysis
The concentration of SDBS/SDS was determined by the methylene blue spectrophotometric method with Daojin UV-2550(P/N206-55501-93) spectrophotometer. The concentration of cadmium ion was analyzed by atomic absorption spectrometry.
4 RESULTS AND DISCUSSION
4.1 Effect of surfactant species and concentration
The surfactant was added into 1000mL solution with the cadmium ions concentration of 100mg/L and the trans-membrane pressure of 0.07MPa and the pH of solution 5. The result is shown in Figs.3 and 4.
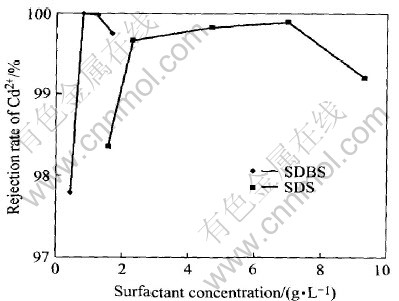
Fig.3 Effect of surfactant concentration on rejection of Cd2+

Fig.4 Effect of surfactant concentration on permeate flux and secondary resistance
As shown in Fig.3, the efficient removal of Cd2+ from aqueous solution is achieved by MEUF technology with SDBS/SDS as surfactant. The rejection rate of Cd2+ reaches 97.8% when the concentration of SDBS amounts to 0.42-1.67g/L(1-4CMC) and the rejection rate of Cd2+ reaches 99.2% when the concentration of SDS amounts to 2.30-9.33g/L. In the above condition, surfactant concentration is close to or exceeds its critical micelle concentration, micelles are already formed, and Cd2+ could be efficiently removed. Compared with SDS, the concentration of SDBS is only 15% of SDS at the same rejection rate of Cd2+. Less dosage of surfactant will cut down the cost for separation. There are two standards for surfactant selection in micellar-enhanced ultrafiltration technology. Firstly, surfactant must be macromolecule. The diameter of micelle must be big enough for large molecular mass cut-off membrane in order to get efficient rejection of Cd2+. Secondly, in order to reduce the dosage of surfactant and the concentration in percolate, the CMC of surfactant must be low enough. Considering the above two standards, SDBS is better than SDS in MEUF for the cadmium ions removal.
The rejection of Cd2+ increases to above 99.9% along with the increase of the concentration of SDS. At the same time, the secondary resistance increases, so the permeate flux decreases gradually. It is because when surfactant concentration increases, big mass of micelle with Cd2+ block membrane forms due to a great deal of micelle in the aqueous solution. Thus the secondary resistance is enhanced, and the permeate flux is declined. Therefore, surfactant concentration should be condign and its critical micelle concentration is the better value. There are enough micelles in aqueous solution but not excessive, the secondary resistance is not too great and the permeate flux is not too small.
4.2 Effect of operating time
The surfactant was added into 1000mL solution with the cadmium ions concentration of 100mg/L, the trans-membrane pressure of 0.07MPa, the concentration of SDBS 0.42g/L and the pH of solution 5. The aqueous solution containing cadmium was ultrafiltrated for 50min. This result is shown in Fig.5.

Fig.5 Effect of operating time on permeate flux and secondary resistance
With the time passing by, the secondary resistance increases and the permeate flux decreases gradually. It is because of the concentration polarization on the surface of membrane. When the contamination on the surface of membrane cumulates to a certain degree, the increase of the secondary resistance slows down and levels off, then the secondary resistance and the permeate flux keep steady. As shown in Fig.5, micellar-enhanced ultrafiltration becomes steady in 20min.
4.3 Influence of trans-membrane pressure
Initial conditions were set to 0.42g/L of the SDBS concentration and 100mg/L of the cadmium concentration and the trans-membrane pressure increasing from 0.04 to 0.10MPa.The value of pH was 5. As shown in Figs.6 and 7, the Trans-permeate pressure has little effect on the rejection of Cd2+. When the trans-membrane pressure is doubled, the rejection of Cd2+ is invariant. At the same time, the permeate flux increases and the secondary resistance decreases. The permeate flux and the secondary resistance are not distinct linear. The increase of the permeate flux and the decrease of the secondary resistance are limited. When the increase comes to horizon, the flux and the resis-tance keep invariant. The concentration of micelles

Fig.6 Effect of trans-membrane pressure on rejection rate of Cd2+
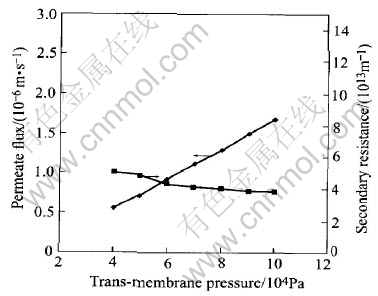
Fig.7 Effect of trans-membrane pressure on permeate flux and secondary resistance
on the surface of membrane increases with the pressure. Gel layer has been formed when micelle concentration on the surface of membrane comes up to gel concentration. If the pressure continues to increase, the gel layer becomes compact, and the secondary resistance increases.
4.4 Effect of pH
In order to study the effect of pH on solution in MEUF process, the experimental condition was set to 0.42g/L(SDBS) or 2.30g/L(SDS) of surfactant concentration and 0.08MPa of the trans-membrane pressure . The concentration of cadmium was 100mg/L. Then solution pH was adjusted from 3 to 13.
As shown in Fig.8, solution pH affects the rejection of Cd2+ directly. pH of solution not only affects the existing form of Cadmium ions but also changes the CMC of surfactant. It plays important role in MEUF process. In SDBS solution, the rejection of Cd2+ increases rapidly when the solutions pH changes from acidic to alkaline. The rejection of Cd2+ reaches 98% when solution pH is 9. There is a great deal of H+ in acidic aqueous solution, and the free H+ adsorbed on micelles surface competes with Cd2+. Thereby, the adsorption of Cd2+ is restrained. Cd2+ becomes hydrate deposition in alkaline solution. In SDS solution, the rejection of Cd2+ changes with solutions pH. The rejection rate of Cd2+ is very high (over 99%) in acidic or alkaline solution, while that in neutral solution is only 80%.

Fig.8 Effect of pH on rejection of Cd2+
Both the permeation flux and the secondary resistance change with pH (see Figs.9 and 10). In SDBS solution, the permeation flux decreases with the increase of solution pH, and the secondary resistance increases with the increase of solution pH. Improvement of the rejection rate of Cd2+ enhances the concentrated polarization on membrane surface. In SDS solution, when solution pH increases, the permeation flux increases firstly and then decreases, while the secondary resistance decreases firstly and then increases.

Fig.9 Effect of pH on secondary resistance
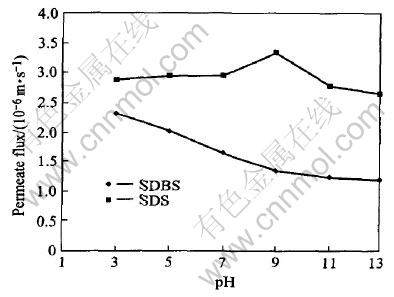
Fig.10 Effect of pH on permeate flux
Anyhow, further studies are in demand for the influencing mechanism of pH on the rejection of cadmium ions, permeation flux and secondary resistance.
4.5 Effect of electrolyte (NaCl)
The existence of electrolyte (NaCl) in solution affects the rejection of Cd2+. The surfactant concentration was set to 0.42g/L(SDBS) and the trans-membrane pressure was set to 0.08MPa. The concentration of cadmium was 100mg/L and pH of solution was 5. Then the concentration of NaCl was adjusted from 10mmol/L to 100mmol/L. The result is shown in Fig.11.

Fig.11 Effect of electrolyte concentration on rejection rate of Ca2+
When the concentration of electrolyte (NaCl) increases, the rejection rate of Cd2+ decreases gradually. Electrolyte in solution competes with concomitant Cd2+, and the Cl- anion of electrolyte reacts with Cd2+.
5 CONCLUSIONS
Micellar-enhanced ultrafiltration is a new technique combining surfactant and ultrafiltration membrane. This technique can separate cadmium ions efficiently from aqueous solution.
Surfactant species, surfactant concentration and solution pH are important controlling factors, while trans-membrane pressure and the existence of electrolyte (NaCl) are less influential. The results show that SDBS is better than SDS in MEUF when it is used to separate cadmium ions from aqueous solution. When the surfactant concentration is 0.42g/L for SDBS, pH of solution is 5 and trans-membrane pressure is 0.07MPa, the rejection rate of cadmium reaches 97.8%. Based on the above experimental results, micellar-enhanced ultrafiltration with SDBS could be used for the treatment of wastewater containing cadmium.
REFERENCES
[1]Qiu T S, Cheng X X, Hao Z W, et al. Disposal actuality of solution including cadmium[J]. Nonferrous Metal in Sichuan, 2002, 4: 38-41.
[2]Michaels A S. Progress in Separation and Purification[M]. New York: Wiley-Interscience, 1968. 297-333.
[3]Leung P S. In Ultrafiltration Membranes and Applications[M]. New York: Plenum, 1979. 415.
[4]Huang Y, Batchelor B, Koseoglu S S. Crossflow surfactant-based ultrafiltration of heavy metals from waste streams[J]. Separation Science and Technology, 1994, 29(15): 1979-1998.
[5]Xu Z L, Xu H M, Zhai X D. Investigation of remove cadmium and lead from aqueous solution via micellar-enhanced ultrafiltration[J]. Membrane Science and Technology, 2002, 22 (3): 15-20.
[6]Shigendo A, Lourdie P C, Susumu N, et al. Separation of Co(Ⅱ)/Ni(Ⅱ) via micellar enhanced ultrafiltration using organophosphorus acid extractant solubilized by nonionic surfactant[J]. Journal of Membrane Science , 1999, 162: 111-117.
[7]Lyudmila Y, Antonina K, Boris K. Removal of Ni(Ⅱ) irons from wastewater by micellar-enhanced ultrafltration[J]. Desalination, 2002, 144: 255-260.
[8]Juang R S, XU Yong-yan, CHEN Ching-liang. Separation and removal of meteal ions form dilute solutions using micellar-enhanced ultrafiltration[J]. Journal of Membrane Science, 2003, 218: 257-267.
[9]Gzara L. Removal of divalent lead cation from aqueous streams using micellar-enhanced ultrafiltration[J]. Rev Sci Eau , 2000, 13: 289-304.
[10]Lassgmd G, Mahmoud D. Removal of chromate anions by micellar-enhanced ultrafiltration using cationic surfactants[J]. Desalination, 2001, 137: 241-250.
[11]Xu Z L, Zhang Y F. Investigation of complexatio-ultrafiltration-electrolysis compositive technology disposed waste water including heavy metal[J]. Membrane Science and Technology, 2003, 23: 141-144.
[12]Shigendo A A, Li Y B, Hiroshi T B. Micellar-enhanced ultrafiltration of gold(III) with nonionic surfactant[J]. Journal of Membrane Science, 1997, 133: 189-194.
[13]Baek K, Kim B K, Cho H J,et al. Removal characteristics of anionic metals by micellar-enhanced ultrafiltration[J]. Journal of Hazardous Materials, 2003, 99: 303-311.
[14]Tung C C, Yang Y M. Removal of copper ions and dissolved phenol from water using micellar-enhanced ultrafiltration with mixed surfactants[J]. Waste Manegement, 2002, 1222: 695-701.
[15]Aoudia M, Allal N. Dynamic micellar enhanced ultrafiltration: use of anionic (SDS)-nonionic(NPE) system to remove Cr3+ at low surfactant concentration[J]. Journal of Membrane Science, 2003, 217: 181-192.
[16]Shi J, Yuan Q, Gao C K. Membrane Technology Enchiridion[M]. Beijing: Chemical Industy Publishing Company, 2001. 1-33.
(Edited by LONG Huai-zhong)
Foundation item: Project (50225926) supported by the National Foundation for Distinguished Young Scholars; Project (20020532017) supported by the Doctoral Foundation of Ministry of Education of China; Project (2003AA644010) supported by the National High-tech Research Program of China
Received date: 2004-09-23; Accepted date: 2004-12-23
Correspondence: ZENG Guang-ming, Professor; Tel: +86-731-8822754; E-mail: zgming@hnu.cn