焙烧-浮选富集石煤中的钒
来源期刊:中国有色金属学报(英文版)2017年第1期
论文作者:刘春 张一敏 包申旭
文章页码:197 - 203
关键词:含钒石煤;焙烧脱碳;矿物学;选择性磨矿;浮选
Key words:vanadium-bearing stone coal; roasting decarburization; mineralogy; preferential grinding; flotation
摘 要:在石煤工艺矿物学的基础上,采用焙烧-浮选新工艺富集石煤中的钒。新工艺包括焙烧脱碳、选择性磨矿、脱泥和浮选4个关键步骤。在焙烧脱碳过程中,550 °C焙烧脱碳能有效避免原矿中的碳质物和焙烧中方解石分解生成游离CaO对浮选产生的不利影响。通过选择性磨矿,高耗酸性矿物在中间粒级富集,而云母在细粒级和粗粒级中富集。浮选得到的最终精矿V2O5的品位为1.07%,回收率为83.30%。此外,最终精矿的钒浸出率相比于脱碳样提高了13.53%。石煤原矿在550 °C焙烧脱碳是可行的,对云母浮选几乎没有不利影响。通过对石煤中钒的浮选预富集,可以降低提钒的处理量、酸耗量和生产成本。
Abstract: A new process for vanadium recovery from stone coal by roasting-flotation was investigated based on the mineralogy. The process comprised four key steps: decarburization, preferential grinding, desliming and flotation. In the decarburization stage, roasting at 550 °C effectively avoided the negative effect of the carbonaceous materials in raw ore and generation of free CaO from calcite decomposition during roasting. Through preferential grinding, the high acid-consuming minerals were enriched in the middle fractions, while mica was enriched in the fine and coarse fractions. Through flotation, the final concentrate can be obtained with V2O5 grade of 1.07% and recovery of 83.30%. Moreover, the vanadium leaching rate of the final concentrate increased 13.53% compared to that of the feed. The results reveal that the decarburization by roasting at 550 °C is feasible and has little negative impact on mica flotation, and vanadium recovery from stone coal is conducive to reducing handling quantity, acid consumption and production cost.
Trans. Nonferrous Met. Soc. China 27(2017) 197-203
Chun LIU1, Yi-min ZHANG1,2,3, Shen-xu BAO1,3
1. College of Resources and Environmental Engineering, Wuhan University of Technology, Wuhan 430070, China;
2. College of Resources and Environmental Engineering, Wuhan University of Science and Technology, Wuhan 430081, China;
3. Hubei Collaborative Innovation Center for High Efficient Utilization of Vanadium Resources, Wuhan 430070, China
Received 11 November 2015; accepted 4 May 2016
Abstract: A new process for vanadium recovery from stone coal by roasting-flotation was investigated based on the mineralogy. The process comprised four key steps: decarburization, preferential grinding, desliming and flotation. In the decarburization stage, roasting at 550 °C effectively avoided the negative effect of the carbonaceous materials in raw ore and generation of free CaO from calcite decomposition during roasting. Through preferential grinding, the high acid-consuming minerals were enriched in the middle fractions, while mica was enriched in the fine and coarse fractions. Through flotation, the final concentrate can be obtained with V2O5 grade of 1.07% and recovery of 83.30%. Moreover, the vanadium leaching rate of the final concentrate increased 13.53% compared to that of the feed. The results reveal that the decarburization by roasting at 550 °C is feasible and has little negative impact on mica flotation, and vanadium recovery from stone coal is conducive to reducing handling quantity, acid consumption and production cost.
Key words: vanadium-bearing stone coal; roasting decarburization; mineralogy; preferential grinding; flotation
1 Introduction
In China, stone coal is a specific vanadium-bearing resource. The gross reserve of vanadium in terms of V2O5 in stone coal is 118 million tons, which accounts for over 87% of the domestic reserve of vanadium [1,2]. Hence, various vanadium extraction techniques from stone coal are investigated. Generally, these techniques involve long processes like roasting, acid leaching, ion purification, precipitation and calcination [3-6]. However, due to the low vanadium grade, complex chemical composition and various occurrences of vanadium of stone coal, vanadium extraction from stone coal is commonly confronted with the problems of enormous ore handling quantity, high acid consumption and high production cost [7].
For the sake of relieving these problems, pre-concentration of vanadium in stone coal before leaching is an effective method. ZHAO et al [8] took gravity separation to pre-concentrate vanadium from stone coal. Although the V2O5 loss was low in gravity separation stage, the yield of final tailings was not high and the separation for the acid-consuming minerals, especially calcite, was not satisfactory. In order to obtain satisfactory separation results, WANG et al [9] chose flotation for beneficiation due to its high handling capacity and high selectivity, and obtained satisfactory flotation results as the stone coal was weathered. Under that condition, little carbon existed in the raw ore and the chemical composition and mineral texture were comparatively simple, which was easy to obtain relatively higher flotation efficiency and better separation results. However, most stone coal in China exists as primary ore, where the carbon content usually ranges from 8% to 12%, and the chemical composition and mineral texture are rather complex. The carbonaceous materials are disseminated among various minerals such as oxides, carbonates, silicates and sulfides, which closely coexist as fine-grained particles. Due to the coating of carbonaceous materials on the surfaces of mineral particles, the floatability differences among different minerals decrease significantly and flotation separation effect is not satisfactory. Even for the severely metamorphic stone coal that carbon mainly exists as graphite, the negative effect of carbon on flotation of stone coal still resulted in a long process, huge reagent consumption and production cost, so technique of decarburization by roasting before floatation was developed [10-13]. Usually the roasting temperature was set over 650 °C so as to remove the carbon quickly without considering the negative effect on flotation. Our previous research [14] found that, with the increase of roasting temperature, reactions like the oxidation of pyrite, combustion of carbon and calcite decomposition occurred successively in vanadium-bearing stone coal. At 600-700 °C, carbon and pyrite disappeared, and octahedral structure in mica was not damaged, while the remaining free CaO after sulfur-fixation of calcite made the pH value and concentration of Ca2+ increase in flotation pulp. Under that condition, it is not conducive to flotation because the fine particles coagulated in the non-selective state, and much fatty acid was consumed, as well as the quartz was activated.
Given that the negative effect of carbonaceous materials and free CaO on flotation should be balanced, reasonable roasting temperature is important for optimizing the flotation process. The focus of this study is to determine a simple and reasonable flotation process for typical primary vanadium-bearing stone coal at relatively low roasting temperature (550 °C). The flotation results are interpreted within the perspective of the effect of the mineralogy on the flotation process and vise versa.
2 Experimental
2.1 Materials and reagents
The vanadium-bearing stone coal used in this study was obtained from Hubei Province, China. The raw ore was firstly crushed to below 2 mm in size with two-stage jaw crusher and one-stage roll crusher. The crushed ore was blended and then split into 200 g samples for mineralogy and flotation tests. The analytical grade reagents used in this study and their abbreviations are listed in Table 1.
Table 1 Reagents used in flotation

2.2 Procedure
The crushed raw ore was firstly decarbonized in a SXZ-10-B muffle furnace at 550 °C for 90 min and then wet-ground in a XMB-70 laboratory rod mill at 50% solids, until a particle size distribution of 84.62% passing 74 μm was achieved. The rod mill product was subjected to desliming by free settling and then the flotation tests was conducted in a 0.5 L flotation cell at an agitation speed of 1992 r/min, in which pH regulator, depressant and collector were added. Two-stage flotation experiments were carried out containing reverse and direct flotation. The detailed conditions and process flowsheet are given in Fig. 1. The flotation products contained slime, V concentrate, tailings 1 and 2. The slime and V concentrate are merged into final concentrate, and the rest are rejected as final tailing. The vanadium of preferential grinding product and final concentrate were leached by 5% CaF2 and 15% (volume fraction) H2SO4 at 95 °C for 6 h.
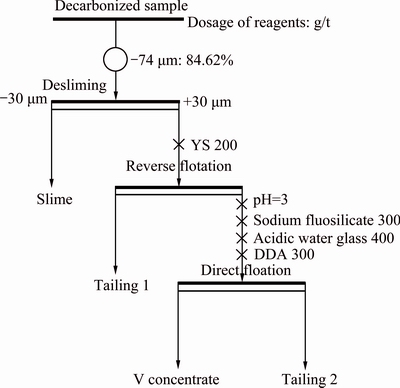
Fig. 1 Flotation conditions and process flowsheet of decarbonized sample
2.3 Test methods
The vanadium grade was measured by Test Methods of Vanadium in Coal Standard (GB/T 19226-2003). X-ray diffraction (XRD) analysis was conducted by D/Max-IIIA X-ray diffractometer with Cu Kα radiation, voltage 40 kV, current 30 mA and at the scanning rate of 15 (°)/min from 5° to 70°. The phases were identified by comparison of the peak positions and d values with data published by the international centre for diffraction data (ICDD). The chemical analysis was performed with the Xios advanced X-ray fluorescence (XRF) analyzer. Detailed mineralogy of the raw ore and decarbonized samples was investigated using Leica DMLP polarization microscope and quantitative evaluation of minerals by scanning electron microscopy (QEMSCAN). Valence distribution was measured on ZDJ-4A automatic potentiometric titrimeter by ammonium ferrous sulfate titration method [15]. Vanadium chemical phase analysis was carried out according to the sequential extraction procedures [16]. Sizing analysis was conducted on rod mill product using laboratory wet screening and classification by free setting. Coarse particles (>38 μm) were classified by wet screening and the fine particles were classified by free setting.
3 Results and discussion
3.1 Mineralogy of raw ore and decarbonized sample
3.1.1 Chemical composition
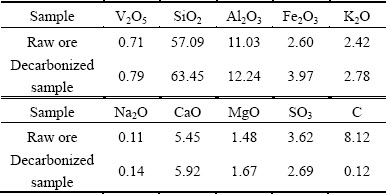
The chemical composition of stone coal is shown in Table 2. It can be seen that the main components in the raw ore are SiO2 and Al2O3, but the content of V2O5 is only 0.71%. As the content of CaO in the raw ore is over 5%, this ore belongs to high-calcium vanadium-bearing stone coal. The high content of CaO not only severely decreases the water leaching rate of vanadium in the traditional technique of NaCl roasting-water leaching, but also increases the acid consumption in the process of acid leaching. Therefore, the CaO should be removed in the reverse flotation.
Table 2 Chemical composition of raw ore and decarbonized sample (mass fraction, %)
3.1.2 Mineral composition
The XRD pattern of the raw ore (Fig. 2) indicates that the main mineral phases are quartz, mica and calcite. In addition, feldspar, pyrite and kaolin exist as the accessory minerals. The mineral composition and content (Table 3) are obtained based on the XRD analysis, microscopy and chemical composition analysis. The results indicate that reducing minerals like coal and pyrite, disappeared in the decarbonized sample, while pyrite was changed into hematite and some decomposed calcite was converted into anhydrite. For decarbonized sample, calcite and hematite belong to high acid consuming minerals, which make it difficult to regulate pH value for mica flotation under acidic condition. Therefore, it is necessary to remove them before the mica flotation, especially calcite because of its much easier dissolution in acid solution compared to that of hematite.
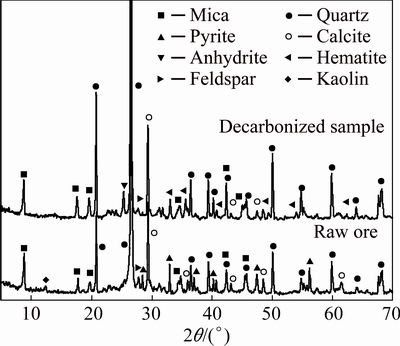
Fig. 2 XRD patterns of raw ore and decarbonized sample
3.1.3 Occurrence of vanadium
The result of vanadium valence distribution measured by potentiometric titration measurement shows that V(III) is dominant which accounts for 79%, and V(IV) accounts for 21%, while V(V) is undetected. Vanadium chemical phase analysis (Table 4) reveals that vanadium mainly exists in silicate, but little in calcite and iron oxide. Previous studies showed that V(III) and V(IV) usually substitutes for Al(III) in the crystal lattice of mica type minerals in the form of isomorphism [3]. Hence, the major gangue minerals are quartz, calcite, feldspar and pyrite, and the main target mineral is mica in the beneficiation process.
Table 3 Mineral composition of raw ore and decarbonized sample (mass fraction, %)

Table 4 Chemical phase analysis of vanadium in raw ore (mass fraction, %)

3.1.4 Lithological feature of decarbonized sample
The mineralogy of the raw ore is complex with several mineral phases including a variety of silicates, oxides, carbonates as well as sulfides. Besides that, the raw ore texture is also complicated. The micro disseminated carbonaceous materials cover on the surface of various minerals especially the fine clay minerals. Mica usually presents in the form of flake and strip, and it is classified into muscovite and illite according to the particle size and associated minerals. The muscovite presents in the form of flake or strip, and it is often associated with quartz and pyrite, and some is even locked in quartz, which makes it difficult to break and recover. The average size of muscovite is 30-45 μm. Small amount of very fine stripy illite particles are generally less than 5 μm and closely associated with kaolin, carbon, fine-grained quartz and feldspar. Most calcite is irregular granular and closely associated with coal, clays and quartz, and some even is locked in quartz vein. Pyrite is associated with quartz and muscovite in the form of regular and semi-regular particle shape. There are three main types of quartz based on the particle size and associated minerals. The vein quartz in the size of 70 μm is often associated with calcite, whereas the lenticular quartz displaying aggregate structure in the size of 20-40 μm is associated with muscovite. The final type whose size is nearly 10 μm is closely associated with clays and carbon. As the composition and texture of the raw ore are so complex, it is difficult to obtain satisfactory flotation result without high enough grinding fineness.
In order to remove the negative effect of carbonaceous materials on flotation and make full use of thermal energy for subsequent leaching, roasting decarburization is necessary for the raw ore before flotation. The changes of chemical and mineral constituents between the decarbonized sample and raw ore are shown in Tables 2 and 3. The results indicate that most carbon was removed, while pyrite was changed into hematite and some decomposed calcite was changed into anhydrite, whereas the free CaO was not found and the octahedral structure of mica was not damaged in the decarbonized sample. QEMSCAN analysis (Table 5) was performed on the decarbonized sample to determine the average grain diameters of minerals. It reveals that minerals are finely disseminated in the decarbonized sample, and it is difficult to separate valuable mineral mica from the associated gangue minerals. What we should note here is that muscovite usually presents in the form of thin strip or flake, so the average mica size here refers to the length of the vein.
3.2 Preferential grinding
Comminution of complex ores is typically necessary to liberate the sought after minerals to allow for their selective recovery processes such as flotation. Preferential grinding of minerals is a common phenomenon in the process of grinding, which is affected by many factors [17]. The internal causes are the differences of the dynamic nature among minerals, and the external causes include applying way of crushing machine, the intensity of the force, mechanical properties, operating conditions, etc. Table 5 shows the obvious differences of average size and Mohs hardness among minerals. From the aspect of mineralogy, the mica is presented as flake or stripy, while the gangue minerals generally presented as irregular or angular grains, which is positive to the preferential grinding in the rod mill [8]. Rod milling can obtain not only even granularity distribution with less content of slime, but also relatively greater size of mica at the same grinding fineness. The reasonable grinding fineness was determined as 84.62% passing 74 μm based on the QEMSCAN analysis of liberating rule occurring in the grinding of mica and gangue minerals.
Composition analysis and yield of each size fraction of preferential grinding product are shown in Fig. 3. Through preferential grinding, the element contents vary with each size fraction. The flake muscovite is enriched in the coarse fractions (>74 μm), and the fine stripy illite is concentrated in the fine fractions (<30 μm). The grade of CaO distributes the characteristics of high intermediate and low at two ends, and the CaO grade of the fine fractions is relatively higher than that of the coarse because the calcite with low Mohs hardness is easy to be ground finely. The grade of TFe also exhibits similar phenomenon because the brittleness of hematite is the dominant factor rather than hardness. Though the preferential grinding is effective, the yield of fine fractions is still relatively high, which may have a negative impact on the subsequent flotation. Hence, the desliming before flotation is really necessary.
3.3 Mica association and liberation
Association and liberation characteristics of mica in the preferential grinding product were identified by QEMSCAN. It was observed that mica was only about 74% liberated (over 90% surface exposed) in the grinding product and generally associated with quartz, hematite, calcite, apatite and feldspar (Fig. 4) in the form of muscovite. The association of muscovite with quartz and feldspar implies that depression of quartz and feldspar in the direct flotation stage would cause some reduction in V2O5 recovery. Besides, hematite of which average size is less than 20 μm is often associated with the fine mica, so it is more difficult to be liberated and recovered compared with calcite from the aspect of average size and association with mica. Therefore, more attention should be paid to calcite rather than hematite in the reverse flotation.
Table 5 Average grain diameter and Mohs hardness of minerals


Fig. 3 Composition and yield of each size fraction of preferential grinding product
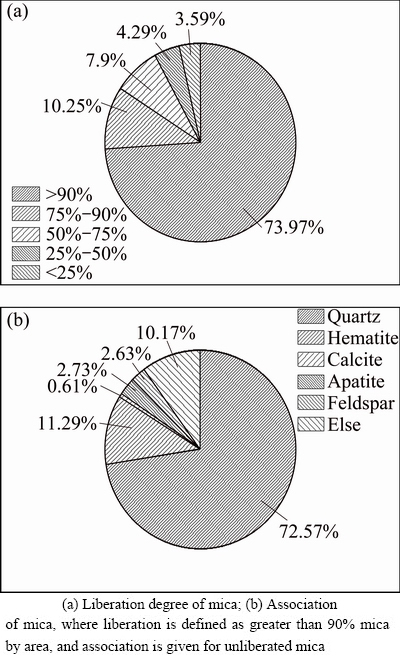
Fig. 4 Association and liberation of mica in preferential grinding product
3.4 Flotation results
As carbonaceous argillaceous shale, stone coal especially decarbonized sample are very apt to be slimed a large amount of secondary slime is produced, which may seriously affect the subsequent flotation [18]. Based on the mineralogical analysis above, a three-stage strategy, desliming followed by reverse and direct flotation, was tested. Because the slime with relatively high V2O5 grade is difficult to make a refinement, it is directly regarded as the concentrate without further refinement. Hence, the concentrate contains both the slime and flotation concentrate. The flotation results of the decarbonized sample are shown in Table 6 and the flotation technological flowsheet of the decarbonized sample is presented in Fig. 5. A total vanadium concentrate (slime and V concentrate ) can be obtained with V2O5 grade of 1.07% and recovery of 83.30%. Besides, the grade and recovery of CaO in tailing 1 were 22.21% and 58.87%. Most of CaO in the decarbonized sample was taken off, while there was only 3.41% loss rate of V2O5 in tailing 1. Therefore, the flotation can not only reject low V2O5 grade minerals as tailings, but also improve the V2O5 grade of the decarbonized sample. The results reveal that the decarburization by roasting at 550 °C is feasible and has little negative impact on the mica flotation, as the flotation technological flowsheet is as simple as common mica flotation [9]. In order to investigate the effectiveness of flotation, acid leaching of the final concentrate and decarbonized sample was conducted, respectively. The vanadium leaching rate of final concentrate increased 13.53% from 45.43% to 58.96% compared to that of the feed. The results above reveal that vanadium recovery from stone coal is conductive to reduce handling quantity, acid consumption and production cost.
Table 6 Flotation results of decarbonized sample (mass fraction, %)
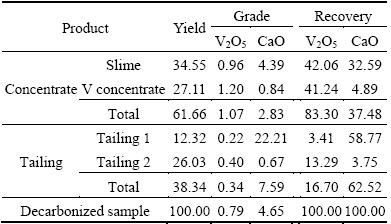

Fig. 5 Flotation technological flowsheet of decarbonized sample
4 Conclusions
1) Based on the mineralogical study on the typical primary vanadium-bearing stone coal, it can be found that various minerals closely coexist as fine-grained particles in the raw ore and the carbonaceous materials are disseminated among them. Hence, roasting decarburization at 550 °C is adopted before flotation to avoid the negative effect of carbon and free CaO on flotation.
2) As the mica presents the flake or stripy morphology, the rod milling exhibits good selectivity to the feed. It can not only obtain even granularity distribution, but also enlarge the composition differences among each size fraction. Through preferential grinding, the fine stripy illite is enriched in the fine fractions, which is beneficial to recovery by desliming.
3) Through roasting-flotation, the final concentrate (slime and V concentrate) can be obtained with V2O5 grade of 1.07% and recovery of 83.30%. Apart from that, 38.34% of the decarbonized sample and 62.52% of CaO can be taken off. Moreover, the vanadium leaching rate of the final concentrate increased 13.53% compared to that of the feed. This study reveals that the decarburization by roasting at 550 °C is feasible and has little negative impact on the mica flotation, as well as vanadium recovery from stone coal is conducive to reduce handling quantity, acid consumption and production cost.
References
[1] BIN Zhi-yong. Progress of the research on extraction of vanadium pentoxide from stone coal and market of the V2O5 [J]. Hunan Nonferrous Metals, 2006, 22(1): 16-20. (in Chinese)
[2] ZHAO Yun-liang, ZHANG Yi-min, BAO Shen-xu, LIU Tao, BIAN Ying, JIANG Mou-feng, LIU Xiang. Loose-stratification model in separation process for vanadium pre-concentration from stone coal [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(2): 528-535.
[3] ZHAO Yun-liang, ZHANG Yi-min, BAO Shen-xu, LIU Tao, BIAN Ying, LIU Xiang, JIANG Mou-feng. Separation factor of shaking table for vanadium pre-concentration from stone coal [J]. Separation and Purification Technology, 2013, 115: 92-99.
[4] LI Cun-xiong, WEI Chang, DENG Zhi-gan, LI Min-ting, LI Xing-bin, FAN Gang. Recovery of vanadium from black shale [J]. Transactions of Nonferrous Metals Society of China, 2012, 20(S1): s127-s131.
[5] LONG Si-si, ZHANG Guo-fan, FENG Qi-ming, OU Le-ming, LU Yi-ping. Desiliconisation of alkaline leaching solution of roasted stone coal with carbonation method [J]. Transactions of Nonferrous Metals Society of China, 2012, 20(S1): s132-s135.
[6] LI Min-ting, WEI Chang, FAN Gang, LI Cun-xiong, DENG Zhi-gan, LI Xin-bin. Pressure acid leaching of black shale for extraction of vanadium [J]. Transactions of Nonferrous Metals Society of China, 2012, 20(S1): s112-s117.
[7] ZHANG Yi-min, BAO Shen-xu, LIU Tao, CHEN Tie-jun, HUANG Jing. The technology of extracting vanadium from stone coal in China: History, current status and future prospects [J]. Hydrometallurgy, 2011, 109(1): 116-124.
[8] ZHAO Yun-liang, ZHANG Yi-min, LIU Tao, CHEN Tie-jun, BIAN Ying, BAO Shen-xu. Pre-concentration of vanadium from stone coal by gravity separation [J]. International Journal of Mineral Processing, 2013, 121: 1-5.
[9] WANG Li, SUN Wei, LIU Run-qing, GU Xiao-chuan. Flotation recovery of vanadium from low-grade stone coal [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(4): 1145-1151.
[10] WU Hui-ling, ZHAO Wei, LI Min-tin, DENG Gan-zhi, GE Huai-hua, WEI Chang. New craft study on enriching vanadium by means of priority coal flotation from high carbon stone-coal [J]. Journal of the Chinese Rare Earth Society, 2008, 26(8): 530-533. (in Chinese)
[11] YE Guo-hua, ZHANG Shuang,HE Wei,TONG Xiong,WU Ning. Process mineralogy characteristics of stone coal and its relationship to vanadium extraction [J]. Chinese Journal of Rare Metals, 2014, 38(1): 146-157. (in Chinese)
[12] BIAN Ying, ZHANG Yi-min, REN Liu-yi, BAO Shen-xu, ZHAO Yun-liang, LIU Xiang. New process for calcite flotation from high calcium and carbon-type vanadium-bearing stone coal [J]. Chinese Journal of Rare Metals, 2014, 38(4): 693-702. (in Chinese)
[13] LI Mao-lin, LIU Peng. Exploratory research on pre-enrichment of Hubei Tongshan stone coal through beneficiation [J]. Nonferrous Metals Mineral Processing Section, 2014(3): 45-49. (in Chinese)
[14] LIU Chun, ZHANG Yi-min, BAO Shen-xu, REN Liu-yi, LIU Xiang. Influence of decarburization by roasting on changes of mineral phases and flotation behavior of vanadium-bearing stone coal [J]. Chinese Journal of Rare Metals, 2016, 40(8): 816-824. (in Chinese)
[15] BAO Shen-xu, ZHANG Yi-min, HUANG Jing., YANG Xiao, HU Yang-jia. Determination of vanadium valency in roasted stone coal by separate dissolve-potentiometric titration method [J]. Materials Research Society Symposium Proceedings, 2011, 1380: 98-103.
[16] BIAN Ying., ZHANG Yi-min, BAO Shen-xu, ZHAO Yun-liang. Analytical method for vanadium occurrence state in stone coal and corresponding chemical explanation [J]. Mining and Metallurgical Engineering, 2013, 33(6): 62-67. (in Chinese)
[17] WEI Xin-chao, HAN Yue-xin, YIN Wan-zhong, ZHAI Yu-chun, TIAN Yan-wen, CHEN Bing-chen. Study on the necessity and flexibility of selective grinding for bauxite [J]. Metal Mine, 2001(10): 29-31. (in Chinese)
[18] HU Xi-geng, HUANG He-wei, MAO Ju-fan. Theory and technology of flotation [M]. Beijing: Science Press, 1991. 390-395. (in Chinese).
刘 春1,张一敏1,2,3,包申旭1,3
1. 武汉理工大学 资源与环境工程学院,武汉 430070;
2. 武汉科技大学 资源与环境工程学院,武汉 430081;
3. 钒资源高效利用湖北省协同创新中心,武汉 430070
摘 要:在石煤工艺矿物学的基础上,采用焙烧-浮选新工艺富集石煤中的钒。新工艺包括焙烧脱碳、选择性磨矿、脱泥和浮选4个关键步骤。在焙烧脱碳过程中,550 °C焙烧脱碳能有效避免原矿中的碳质物和焙烧中方解石分解生成游离CaO对浮选产生的不利影响。通过选择性磨矿,高耗酸性矿物在中间粒级富集,而云母在细粒级和粗粒级中富集。浮选得到的最终精矿V2O5的品位为1.07%,回收率为83.30%。此外,最终精矿的钒浸出率相比于脱碳样提高了13.53%。石煤原矿在550 °C焙烧脱碳是可行的,对云母浮选几乎没有不利影响。通过对石煤中钒的浮选预富集,可以降低提钒的处理量、酸耗量和生产成本。
关键词:含钒石煤;焙烧脱碳;矿物学;选择性磨矿;浮选
(Edited by Yun-bin HE)
Foundation item: Project (2015BAB03B05) supported by the National Key Technology R&D Program during the “12th Five-year Plan” Period, China; Project (51404177) supported by the National Natural Science Foundation of China
Corresponding author: Yi-min ZHANG; Tel: +86-27-87882128; E-mail: zym126135@126.com
DOI: 10.1016/S1003-6326(17)60022-0

