
Anti-oxidation behavior of chemical vapor reaction SiC coatings on different carbon materials at high temperatures
YANG Xin(杨 鑫)1, HUANG Qi-zhong(黄启忠)1, ZOU Yan-hong(邹艳红)2,
CHANG Xin(常 新)1, SU Zhe-an(苏哲安)1, ZHANG Ming-yu(张明瑜)1, XIE Zhi-yong(谢志勇)1
1. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China;
2. Materials Science and Engineering Post-doctoral Research Center, Central South University,
Changsha 410083, China
Received 21 October 2008; accepted 5 January 2009
Abstract: To protect carbon materials from oxidation, SiC coatings were prepared on carbon/carbon(C/C) composites and graphite by chemical vapor reaction. SEM and XRD analyses show that the coatings obtained are composed of SiC grains and micro-crystals. The influence of different carbon substrates on oxidation behavior of coated samples was investigated, and then their oxidation mechanisms were studied. Oxidation test shows that the SiC coated graphite has a better oxidation resistance than SiC coated C/C composites at high temperatures (1 623 K and 1 823 K). In the oxidation process, the oxidation curves of SiC coated C/C composites are linear, while those of SiC coated graphite follow a quasi-parabolic manner. The oxidation mechanism of the former is controlled by chemical reaction while the latter is controlled by oxygen diffusion based on the experimental results. The variation of oxidation behavior and mechanism of SiC coatings on two kinds of carbon substrates are primarily contributed to their structure differences.
Key words: carbon/carbon composites; graphite; coating; SiC
1 Introduction
Carbon/carbon(C/C) composites are well- established materials with lightweight, high strength, high stiffness, excellent thermal shock and thermal erosion resistance[1-2]. For these unique properties, they become important high-temperature structural materials widely used in fusion reactors, space shuttle, rocket nozzles, hypersonic vehicles and propulsion systems [3-5]. However, the main factor that restricts the C/C composites in high-temperature structural applications is their easy oxidation in an oxygen-containing atmosphere above 723 K[6-7]. Without protection, carbon fibers may react easily with oxygen and rapidly burn away. As a result, erosion of structure and degradation of material properties take place simultaneously.
As one promising material with high melting point (2 873 K) and excellent oxidation resistance, SiC is usually considered as the ideal coating material because it not only has the good compatibility with C/C substrates, but also can form self-healed silica glass with low oxygen permeability, which can effectively prevent oxygen diffusing into the substrates[8-9].
For applying SiC coating to the surface of C/C composites, many methods such as pack cementation [10], chemical vapor deposition(CVD)[11], laser- induced chemical decomposition(LICD)[12], plasma spraying[13] and slurry-sintering[14] have been developed and achieved partial success. Of these reported techniques, CVD, LICD and plasma spraying techniques are complicate to operate because they need to coat each surface of the sample separately[15]. Though the pack cementation technique can coat all layers of surface in one process, it still needs an extra procedure to remove the loose material embedded. Compared with the methods mentioned above, the slurry method is more suitable to prepare SiC coatings on carbon materials for its advantages of low cost, easy operation, and no special requirements to the shape of sample[8, 16]. But unfortunately, this method needs vacuum and high temperature to anneal the sample, and also its reliability of obtaining a uniform coating still needs further verification. Therefore, it is essential and urgent to develop better methods that can prepare dense coatings with excellent oxidation resistance for carbon materials.
In the present study, a novel method of chemical vapor reaction(CVR) was used to prepare dense SiC coatings on C/C composites and graphite. The influence of carbon substrates on oxidation behavior of coated samples was studied. The reasons behind this variation were presented. Also, the oxidation mechanisms of the coated samples at high temperature were investigated.
2 Experimental
Two kinds of carbon materials including C/C composites and graphite were used as substrates in this study. The C/C composites were produced by chemical vapor infiltration in our laboratory, which were 2.5 dimensional materials with a density of 1.72 g/cm3. The graphite with the density of 1.76 g/cm3 and the as-prepared C/C composites were cut into small specimens with a size of 12 mm×10 mm×10 mm. Before the CVR, the specimens were hand-polished using 600 grit SiC paper, then cleaned ultrasonically with ethanol and dried at 393 K for 2 h. The materials used in the CVR were Si sheets (industrial reagent, >99.4%) and SiO2 powders (industrial reagent, >99.2%). After being washed by dilute hydrochloric acid to remove the impurities, the Si sheets and SiO2 powders were placed in a graphite crucible and heated to generate vapor at temperature above their melting points. Meanwhile, the as-prepared specimens were exposed to the mixed vapor to form the coatings. The whole process was conducted in the temperature range of 1 923-2 273 K for 1-3 h, followed by a natural cooling course. Details for preparing the CVR SiC coatings were reported in Ref.[17].
The isothermal oxidation tests of the coated samples were carried out at 1 623 K and 1 823 K in an electrical furnace in air. The effectiveness of the SiC coatings on the oxidation behavior of different carbon substrates was expressed by mass change percentage of the oxidized samples. The morphologies and crystalline structures of the coatings were analyzed by scanning electron microscopy(SEM) and X-ray diffractometry(XRD), respectively.
3 Results and discussion
3.1 Microstructure of coatings
Fig.1 shows the XRD patterns of the SiC coatings on different carbon substrates prepared with CVR. As shown in Fig.1, besides those strong peaks corresponding to SiC, there is no detectable peaks of Si or SiO2. It is therefore concluded that the phase composition of the coating is SiC.. Moreover, no diffraction peaks corresponding to carbon are present in the XRD spectra, which demonstrates that pure SiC coating can be obtained by CVR.
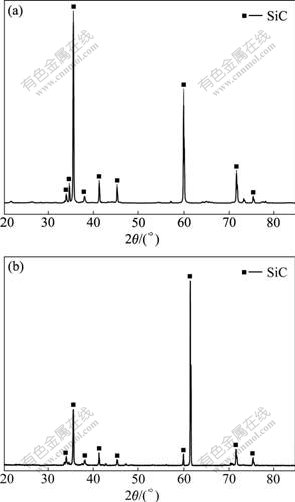
Fig.1 XRD patterns of as-prepared SiC coatings on different carbon substrates: (a) C/C composites; (b) graphite
Typical surface morphologies of the obtained SiC coatings on different carbon substrates are shown in Fig.2. From Fig.2, it can be seen that large SiC grains together with micro-crystals are both present on the coating surfaces, indicating that a similar structure is formed. In spite of this similar surface morphology, the obtained coatings also reveal structural differences to some extent. Compared with the coatings on C/C composites, the SiC coating on graphite is denser and the crystals on the surface are bigger, which is advantageous to improving the oxidation resistance of the coating. While as for the coating that was prepared on C/C composites, visible cracks and holes are formed due to the strong anisotropic nature of the composites. As C/C composites are of porous structure with multiphase composition, the different expansion coefficients between carbon fiber and the pyrolytic carbon, and the anisotropic expansion coefficients of carbon fibers in different directions are responsible for the formation of these defects.

Fig.2 SEM images of SiC coatings on different carbon substrates: (a) C/C composites; (b) Graphite
3.2 Anti-oxidation property of coatings
Fig.3 presents the isothermal oxidation curves of the SiC coated samples in air at 1 623 K. As shown in Fig.3, it is obvious that two kinds of coated samples exhibit a totally different anti-oxidation behavior. In the whole oxidation course, the mass loss of SiC coated C/C increases linearly with oxidation time. After 15 h of oxidation, the mass loss of the coated C/C is up to 8.36%, which suggests that the coating is not dense and the oxygen can directly attack the carbon substrate. In contrast, the SiC coated graphite exhibits excellent oxidation resistance in air at 1 623 K, and gains mass continuously in the whole oxidation process. After 42 h of oxidation, the mass gain of the coated sample is 0.39%, indicating a better oxidation protection of the coating.
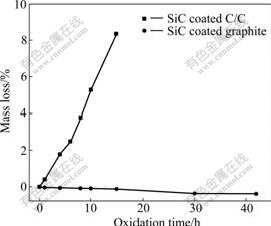
Fig.3 Isothermal oxidation curves of SiC coated samples in air at 1 623 K
Additional oxidation test was also carried out in air at 1 823 K (see Fig.4). Combined with the result in Fig.3, it is noteworthy that similar result is obtained at different oxidation temperatures. For the SiC coated C/C oxidized at 1 823 K, the mass loss as a function of time is still linear, and after 6 h oxidation, the mass loss exceeds 5.60%, which further confirms that the protection ability of the coating is unsatisfactory. With respect to the SiC coated graphite, the mass gain in the oxidation process recurs and no obvious mass loss is observed. After the oxidation at 1 823 K for 60 h, the mass gain of the coated sample is 0.074%, indicating that the as-prepared coating has excellent anti-oxidation resistance at high temperatures. Therefore, based on the above results, it can be concluded that the SiC coated graphite has better oxidation resistance than SiC coated C/C.

Fig.4 Isothermal oxidation curves of SiC coated samples in air at 1 823 K
To investigate the different oxidation behaviors of the coated samples, the microstructure of the samples after oxidation was observed by SEM. Fig.5 displays the surface morphologies of the coated samples after oxidation test at 1 623 K. As shown in Fig.5, it can be seen that SiO2 glass together with micro-cracks are both formed on the coating surfaces. The formation of SiO2

Fig.5 Surface morphologies of coated samples after isothermal oxidation test in air at 1 623 K: (a) C/C composites; (b) Graphite
glass can reduce the oxidation rate and act as a sealant to fill the defects in the coating, which is beneficial to improving the oxidation resistance of the coating. Furthermore, it should be noted that some open holes are also formed on the coating layer on C/C, which is not good for the anti-oxidation purpose of the coating. In addition, it must be pointed out that these defects formed (including cracks and holes) in the coating can develop as diffusion path for oxygen to attack the substrate when they are not fully sealed at high temperature. Theoretically speaking, the less the formed defects, the better the oxidation resistance of the coating will be.
Representative cross-section morphologies of the SiC coating on C/C after oxidation test at 1 623 K are shown in Fig.6. From Fig.6(a), it can be seen that the coating after oxidation is still continuous and uniform, though evident oxidation of substrate is observed. This detectable oxidation sign implies that penetrating defects are formed in the coating, which may be responsible for the mass loss of the coated sample. Meanwhile, the SEM micrograph in higher magnification (Fig.6(b)) reveals that the coating is still integrate and adheres well with the substrate even after long time oxidation, indicating the good bonding ability of the coating prepared with CVR.
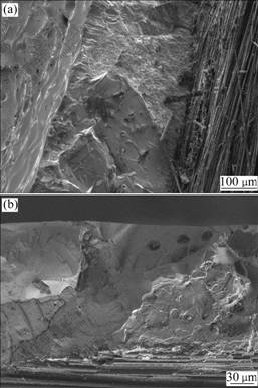
Fig.6 Cross-section morphologies of SiC coating on C/C composites after isothermal oxidation test in air at 1 623 K
Fig.7 shows the surface micrographs of the SiC coatings on different carbon substrates after oxidation test at 1 823 K. Compared with the coating on graphite, the coating on C/C contains more cracks and holes, which is consistent with the results shown in Fig.5. To better understand the oxidation behavior of the two coatings, the cross-section of the coated samples was also examined and their images are shown in Fig.8. As shown in Fig.8(a), it is clear that the SiC coating on C/C composites is not totally dense, and penetrating defects formed in the thinner area of the coating can be verified. The formation of these defects provides the diffusion channels for oxygen to attack the substrate, and thus results in the mass loss of the coated sample. Interestingly and as apparent from Fig.8(a), a big cavity formed under the coating layer is also observed, which further confirms that the limited oxidation protection of the coating is ascribed to the formation of these penetrating defects. Meanwhile, the cross-section image of the SiC coated graphite reveals that no penetrating defects are formed in the coating layer (Fig.8(b)), which suggests that the coating after the oxidation is still dense and the excellent oxidation resistance of the coated sample is mainly contributed to the dense structure of the coating.

Fig.7 SEM images of SiC coatings on different carbon substrates after isothermal oxidation test in air at 1 823 K: (a) C/C composites; (b) Graphite

Fig.8 Cross-section morphologies of SiC coatings on different carbon substrates after isothermal oxidation test in air at 1 823 K: (a) C/C composites; (b) Graphite
3.3 Anti-oxidation mechanism of coatings
According to the oxidation results shown in Figs.3 and 4, it is evident that the relationships between mass change and time of these two kinds of coated samples are different. For the SiC coated C/C, the oxidation curves at 1 623 K and 1 823 K are linear, which indicates that the oxidation mechanism of the coated sample is the chemical reaction of oxygen with the carbon substrate. On the other hand, the SiC coated graphite shows quasi-parabolic oxidation curves at 1 623 K and 1 823 K, indicating that the oxidation is mainly controlled by diffusion and the coating exhibits excellent oxidation resistance at high temperatures.
As reported in previous studies [10, 18-21], the oxidation mechanisms of the SiC coatings at different temperatures (below 2 001 K) were proposed and they can be summarized as: the chemical reaction controlled mechanism, the diffusion controlled mechanism and the reaction-diffusion controlled mechanism. However, the oxidation of the coating is a complicated process and it can be influenced by many factors such as temperature, oxidation environment, microstructure and composition of the coating. So, SiC coatings prepared with various methods under different oxidation conditions usually exhibit different oxidation behaviors and mechanisms. In the oxidation process, the chemical reaction in the coating and the oxygen diffusion inside the coating will occur simultaneously, and thus the oxidation process is controlled by both oxygen diffusion and reaction kinetics at high temperature. This means that the oxidation rate is mainly determined by the competition process between the chemical reaction and oxygen diffusion. Much specifically, when the chemical reaction rate is lower than the diffusion rate, the rate of oxygen diffused inward is higher than that of reaction, and in this case, the oxidation process is controlled by chemical reaction. On the other hand, if the chemical reaction rate is higher than the diffusion rate, the oxygen diffused inside the coating is insufficient for reaction consumed, thus the oxidation process is controlled by oxygen diffusion. Finally, when the diffusion rate approximates to the chemical reaction rate, the oxidation process is influenced by both of them, and thus controlled by them together.
For SiC coated graphite, the coating is dense and the oxygen permeability of the formed SiO2 glass is very low. So, when the cracks on the coating surface are sealed, oxygen can hardly diffuse into the substrate. In this case, the oxidation can only occur in the superficial area of the coating and consequently the oxidation process is controlled by oxygen diffusion. However, for SiC coatings used in oxidizing environment, this oxidation mode is favorable for long time anti-oxidation protection because the substrate can be effectively isolated from oxygen. As concerned with the SiC coated C/C composites, because the penetrating defects such as cracks and holes are formed in the coating, oxygen diffusing through these defects is much easy. Correspondingly, the oxygen diffusion rate in SiC coated C/C is much higher than that of SiC coated graphite. Meanwhile, when the carbon surface is covered with SiC coating, the amount of active carbon atom exposed to air is small, thus the reaction rate is very low and then the oxidation is determined by chemical reaction. For this reason, it is believed that the oxidation rate of the SiC coated C/C at different temperatures is mainly concerned with reaction rate. According to Arrhenius equation: k=Aexp(-E/RT), the oxidation rate at 1 823 K is higher than that at 1 623 K, thus the coated sample shows a higher mass loss rate at 1 823 K.
Based on the above analysis, it can be inferred that the oxidation behavior and mechanism variations of the SiC coatings on two kinds of carbon substrates are primarily contributed to their structure differences. The reasons for the structure differences can be explained as follows. Firstly, the coefficients of thermal expansion(CTE) of C/C composites, graphite and SiC are 1.2×10-6, 3.2×10-6 and 4.6×10-6 K-1, respectively [22-23]. Compared with graphite, the mismatch of CTE between C/C composites and SiC is larger. This means that cracks are more easier to form due to the tensile stress induced by this mismatch. On the other hand, it is well known that C/C composites are of porous structure that consists of carbon fiber and pyrolytic carbon. Thus, during CVR, holes and cracks in the substrate are not fully filled, and defects in the coating are always formed due to the hereditary structure characteristics of the substrate. Finally, in comparison with pyrolytic carbon, the siliconization of carbon fiber is more difficult[17] and thus it can be hardly coated to obtain a continuous coating. For the reasons above, it is reasonable to believe that the formation of defects in the single-layer SiC coating is inevitable, and to improve the oxidation resistance of CVR SiC coating on C/C composites, additional researches that focus on eliminating of these defects are necessary.
4 Conclusions
1) Oxidation protective SiC coatings can be prepared on both graphite and C/C composites by CVR. The coatings obtained are pure SiC and reveal good adherence with the substrates.
2) Oxidation tests performed at 1 623 and 1 823 K show that the SiC coated graphite gains mass continuously, while the SiC coated C/C composites lose mass gradually. The results indicate that the SiC coated graphite has better oxidation resistance than the SiC coated C/C composites. The limited oxidation resistance of the SiC coated C/C composites is mainly contributed to the penetrating defects formed in the coating.
3) In the oxidation process, the oxidation curves of the SiC coated graphite at 1 623 K and 1 823 K are linear, while those of SiC coated graphite follow a quasi- parabolic manner. The oxidation mechanism of the former is controlled by the chemical reaction and the latter is controlled by oxygen diffusion. The different oxidation mechanisms of SiC coatings are also contributed to their structure differences.
Acknowledgements
The authors would like to thank Dr. YANG Li in College of Materials Science and Engineering of Hunan University for assistance with the SEM analysis.
References
[1] SHIMADA S, SATO T. Preparation and high temperature oxidation of SiC compositionally graded graphite coated with HfO2 [J]. Carbon, 2002, 40: 2469-2475.
[2] LI Guo-dong, XIONG Xiang, HUANG Bai-yun, HUANG Ke-long. Structural characteristics and formation mechanisms of crack-free multilayer TaC/SiC coatings on carbon-carbon composites [J]. Trans Nonferrous Met Soc China, 2008, 18(2): 255-261.
[3] FU Qian-gang, LI He-jun, SHI Xiao-hong, LI Ke-zhi, WEI Jian, HUANG Min. Oxidation protective glass coating for SiC coated carbon/carbon composites for application at 1 773 K [J]. Materials Letters, 2006, 60: 431-434.
[4] ZHU Y C, OHTANI S, SATO Y, IWAMOTO N. The improvement in oxidation resistance of CVD-SiC coated C/C composites by silicon infiltration pretreatment [J]. Carbon, 1998, 36: 929-935.
[5] KIM J I, KIM W J, CHOI D J, PARK J Y, RYU W S. Design of a C/SiC functionally graded coating for theoxidation protection of C/C composites [J]. Carbon, 2005, 43: 1749-1757.
[6] SMEACETTO F, SALVO M, FERRARIS M. Oxidation protective multilayer coatings for carbon/carbon composites [J]. Carbon, 2002, 40: 583-587.
[7] LI He-jun, FU Qian-gang, SHI Xiao-hong, LI Ke-zhi, HU Zhi-biao. SiC whisker-toughened SiC oxidation protective coating for carbon/carbon composites [J]. Carbon, 2006, 44: 602-605.
[8] FU Qian-gang, LI He-jun, SHI Xiao-hong, LI Ke-zhi, WANG Chuang, HUANG Min. Double-layer oxidation protective SiC/glass coatings for carbon/carbon composites [J]. Surface and Coatings Technology, 2006, 200: 3473-3477.
[9] YAN Zhi-qiao, XIONG Xiang, XIAO Peng, CHEN Feng, ZHANG Hong-bo, HUANG Bai-yun. A multilayer coating of dense SiC alternated with porous Si-Mo for the oxidation protection of carbon/carbon silicon carbide composites [J]. Carbon, 2008, 46: 149-153.
[10] HUANG Jian-feng, ZENG Xie-rong, LI He-jun, XIONG Xin-bo, FU Ye-wei. Influence of the preparation temperature on the phase, microstructure and anti-oxidation property of a SiC coating for C/C composites [J]. Carbon, 2004, 42: 1517-1521.
[11] CHENG Lai-fei, XU Yong-dong, ZHANG Li-tong, YIN Xiao-wei. Preparation of an oxidation protection coating for C/C composites by low pressure chemical vapor deposition [J]. Carbon, 2000, 38: 1493-1498.
[12] SNELL L, NELSON A, MOLIAN P. A novel laser technique for oxidation resistant coating of carbon-carbon composites [J]. Carbon, 2001, 39: 991-999.
[13] HUANG Jian-feng, LI He-jun, ZENG Xie-rong, LI Ke-zhi, XIONG Xin-bo, HUANG Min, ZHANG Xiu-lian, LIU Ying-lou. A new SiC/yttrium silicate/glass multi-layer oxidation protective coating for carbon/carbon composites [J]. Carbon, 2004, 42: 2356-2359.
[14] ZHAO Juan, WANG Gui, GUO Quan-gui, LIU Lang. Microstructure and property of SiC coating for carbon materials [J]. Fusion Eng Des, 2007, 82: 363-368.
[15] LI He-jun, JIAO Geng-sheng, LI Ke-zhi, WANG Chuang. Multilayer oxidation resistant coating for SiC coated carbon/carbon composites at high temperature [J]. Mater Sci Eng A, 2008, 475: 279-284.
[16] XIAO Lai-rong, YI Dan-qing, YIN Lei, CAI Zhi-gang. Morphology and structure of high temperature MoSi2 coating on niobium [J]. Trans Nonferrous Met Soc China, 2005, 15: 18-22.
[17] LIU Xing-fang, HUANG Qi-zhong, SU Zhe-an, JIANG Jian-xian. Preparation of SiC coating by chemical vapor reaction [J]. Journal of the Chinese Ceramic Society, 2004, 32(7): 906-910. (in Chinese)
[18] FRITZE H, JOJIC J, WITKE T, R?SCHER C, WEBER S, SCHERRER S, WEI? R, SCHULTRICH B, BORCHARDT G. Mullite based oxidation protection for SiC-C/C composites in air at temperatures up to 1 900 K [J]. Journal of European Ceramic Society, 1998, 18: 2351-2364.
[19] GAO Peng-zhao, XIAO Han-ning, WANG Hong-jie, JIN Zhi-hao. A study on the oxidation kinetics and mechanism of three-dimensional (3D) carbon fiber braid coated by gradient SiC [J]. Materials Chemistry and Physics, 2005, 93: 164-169.
[20] HIROSHI H, TAKUYA A, YASUO K, TOSHIO Y. High-temperature oxidation behavior of SiC-coated carbon fiber-reinforced carbon matrix composites [J]. Composites: Part A, 1999, 30: 515-520.
[21] NASLAIN R, GUETTE A, REBILLAT F, GALLET SLE, LAMOUROUX F, FILIPUZZI L, LOUCHET C. Oxidation mechanisms and kinetics of SiC-matrix composites and their constituents [J]. Journal of Materials Science, 2004, 39: 7303-7316.
[22] ZENG Xie-rong, ZHENG Chang-qing, LI He-jun, YANG Zheng. Properties of oxidation resistant MoSi2 coating of carbon/carbon composites [J]. Acta Materiae Composite Sinica, 1997, 14(3): 37-40. (in Chinese)
[23] ZHAO Juan, WANG Gui, LIU Lang. Oxidation resistance property of SiC/Si-MoSi2/MoSi2 coating [J]. Journal of Chinese Society for Corrosion and Protection, 2008, 28(3): 161-165. (in Chinese)
Foundation item: Project(2006CB600901) supported by the National Basic Research Program of China; Projects(50772134, 50802115) supported by the National Natural Science Foundation of China
Corresponding author: HUANG Qi-zhong; Tel: +86-731-88836078; Fax: +86-731-88836081; E-mail: qzhuang@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(08)60404-5
(Edited by YUAN Sai-qian)