锑胁迫下地枇杷的生理变化特性
来源期刊:中国有色金属学报(英文版)2017年第4期
论文作者:柴立元 王勇 杨志辉 H. MUBARAK N. MIRZA
文章页码:939 - 945
关键词:锑;生物量;抗氧化酶活性;地枇杷;叶绿素荧光
Key words:antimony; biomass; antioxidant enzyme activity; F. tikoua; chlorophyll fluorescence
摘 要:采用温室培养试验研究锑对地枇杷生理变化的影响。结果表明,当锑浓度高于30 μmol/L时,地枇杷叶片的生长明显受到抑制,而地枇杷的根和茎在所有的锑浓度下都没有明显的变化,表明地枇杷的叶片比根和茎对锑的毒性更敏感,且在根中锑浓度要高于茎和叶中的。地枇杷通过增加超氧化物歧化酶(SOD)、过氧化物酶(POD)和过氧化氢酶(CAT)活性来减少锑胁迫产生的活性氧(ROS)水平, 但在锑胁迫早期,SOD和CAT比POD的作用更明显。在锑胁迫早期,不同浓度的锑能够在一定程度上增加地枇杷叶绿素的含量,但在锑胁迫后期(实验结束时),叶绿素含量在高浓度锑(450 μmol/L)胁迫下显著降低。在整个培养阶段中,地枇杷最大光量子产量和实际光量子产量值没有显著差异,表明地枇杷在锑浓度为450 μmol/L以下时,光合作用未受明显抑制。地枇杷具有一定的锑耐受能力,可用于锑污染土壤修复。
Abstract: A greenhouse culture experiment was used to evaluate the effects of antimony (Sb) stress on Ficus tikoua (F. tikoua). The results showed that the growth of F. tikoua leaves was significantly inhibited when Sb concentration was higher than 30 μmol/L, and no significantly inhibitory effect of Sb on the roots and stems of F. tikoua was found in all the treatments, implying that leaves were more sensitive to Sb toxicity than roots and stems. Antimony concentration in the roots was higher than that in the stems and leaves. To reduce reactive oxygen species (ROS) level in the F. tikoua, the activities of superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) increased with Sb treatments, but the SOD and CAT were more early active than POD. Although the decrease of chlorophyll content with high Sb treatments (450 μmol/L) was observed at the end of the experiments, the positive impact on chlorophyll content was observed with all the Sb treatments at the early period. No significant difference of the maximum quantum efficiency of PSII and quantum yield of PSII electron transport values with different Sb treatments was observed at the end of this experiment, suggesting that the photosynthesis was not inhibited with Sb concentration below 450 μmol/L. The results implied a certain tolerance to Sb stress for F. tikoua. This meets the essential condition for utilization in Sb contamination environments.
Trans. Nonferrous Met. Soc. China 27(2017) 939-945
Li-yuan CHAI1,2, Yong WANG1, Zhi-hui YANG1,2, H. MUBARAK1, N. MIRZA1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Chinese National Engineering Research Center for Control and Treatment of Heavy Metal Pollution, Central South University, Changsha 410083, China
Received 30 May 2016; accepted 6 January 2017
Abstract: A greenhouse culture experiment was used to evaluate the effects of antimony (Sb) stress on Ficus tikoua (F. tikoua). The results showed that the growth of F. tikoua leaves was significantly inhibited when Sb concentration was higher than 30 μmol/L, and no significantly inhibitory effect of Sb on the roots and stems of F. tikoua was found in all the treatments, implying that leaves were more sensitive to Sb toxicity than roots and stems. Antimony concentration in the roots was higher than that in the stems and leaves. To reduce reactive oxygen species (ROS) level in the F. tikoua, the activities of superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) increased with Sb treatments, but the SOD and CAT were more early active than POD. Although the decrease of chlorophyll content with high Sb treatments (450 μmol/L) was observed at the end of the experiments, the positive impact on chlorophyll content was observed with all the Sb treatments at the early period. No significant difference of the maximum quantum efficiency of PSII and quantum yield of PSII electron transport values with different Sb treatments was observed at the end of this experiment, suggesting that the photosynthesis was not inhibited with Sb concentration below 450 μmol/L. The results implied a certain tolerance to Sb stress for F. tikoua. This meets the essential condition for utilization in Sb contamination environments.
Key words: antimony; biomass; antioxidant enzyme activity; F. tikoua; chlorophyll fluorescence
1 Introduction
Antimony (Sb) is a toxic element with adverse effects on humans and the environment. An increase in Sb mining and smelting processes has resulted in the release of large quantities of Sb, causing serious Sb contamination in local environment [1,2]. Even at low concentration, Sb can cause irreparable damage to the environment [3]. Antimony is known to suppress germination and growth, inhibit photosynthesis, affect membrane structure and permeability, and damage the structure and function of photosystem II (PSII) in plants [4,5]. Research has indicated that the inhibition of photosynthesis is a primary damage mechanism in plants exposed to Sb even at a lower concentration [4,6]. Under Sb stress, the chlorophyll synthesis was inhibited and plastoquinone pool was diminished in plants, with a significant reduction in the total area between the fluorescence induction curves [7]. Also, Sb exposure resulted in more strong inhibition of electron transport both on PSII donor side and acceptor side than the oxygen-evolving complex and light-harvesting pigment- protein complex II [8].
A typical symptom of Sb toxicity is oxidative damage. In addition, Sb can induce the production of reactive oxygen species (ROS), including superoxide radicals (O-2), hydroxyl radicals (·OH) and hydrogen peroxide (H2O2), which react very rapidly with DNA, lipids and proteins to cause cellular damage [9]. The excessive ROS reacts with lipids, proteins and pigments, which results in membrane damage and enzyme inactivation [9]. Antimony toxicity leads to the generation of ROS by inhibiting the electron transport chain in the chloroplast and mitochondria [9,10]. To counter ROS, plants produce antioxidants and antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT).
Ficus tikoua (F. tikoua), an endemic species in China belonging to the Moraceae family, has been recognized as a valuable restoration species and a medicinal source [11,12]. Ficus tikoua has been found wildly growing in the active and abandoned Sb mining areas. During the past decade, research focused on the evaluation of biological activities of F. tikoua [13]. Few research studies have investigated the photosynthetic response of F. tikoua to metal/metalloid stress [14]. Moreover, little is known about the response of F. tikoua to Sb treatment. Thus, the objective of this study is to assess the Sb resistance capacity of F. tikoua in preparation for eventual application to the re-vegetation of Sb-polluted soils.
2 Experimental
2.1 Plant cultivation and Sb treatment
Cuttings of F. tikoua from an antimony mine area in Lengshuijiang city, Hunan province, China (111°29′E, 27°45′N) were grown on water-saturated perlite in a growth chamber in a greenhouse. The cuttings were illuminated with three 25 W fluorescent lamps; the photoperiod was set as 14 h/10 h and the light intensity was 150 μmol/(m2·s) photosynthetically active radiation (PAR). Day/night temperatures of 25 °C/18 °C were applied, and the humidity was 60%-80%. Cuttings were supplied with half-strength Hoagland nutrient solution until all six or seven leaves were completely unfolded. Thereafter, four plants of F. tikoua were carefully transferred to plastic pots that were filled with perlites. Four Sb concentrations (0, 30, 150 and 450 μmol/L) were tested in this study. Antimony was supplied as C4H4KO7Sb·1/2H2O. Each pot was supplied with 50 mL of Sb-containing half-strength Hoagland solution at 5 d intervals. All conditions were the same as those described above.
2.2 Observation of biomass and antimony in plant tissue
After 78 d, the Sb-exposed plants were collected and then rinsed three times with deionized water. The roots, stems and leaves were separately collected, dried at 70 °C and weighted. The plant samples were ground and sieved through a 1 mm sieve and digested with V(HNO3):V(HClO4)=4:1. The Sb concentration in the digested solution was quantified by atomic fluorescence spectrometry (AFS) (Titan AFS-810).
2.3 Assay of antioxidant enzymatic activities and MDA content
Approximately 0.2 g of fresh tissue was homogenized in a pre-cooled mortar with 5 mL precooled sodium phosphate buffer (50 mmol/L, pH 7.8) solution. And then the supernatant was collected for detection of enzyme activity by centrifugation for 20 min at 11000g and 4 °C [10]. The supernatant was used to determine SOD, POD, CAT, MDA and soluble sugar concentration according to the following photo-chemical method.
1) SOD assay was performed according to the method of TANG et al [15], with some modifications. Briefly, 3 mL reaction mixture that contains 0.3 mL of 750 μmol/L nitroblue tetrazolium (NBT), 0.3 mL of 20 μmol/L riboflavin, 0.3 mL of 130 mmol/L methionine, 0.3 mL of 100 μmol/L EDTA-Na2, 1.5 mL of sodium phosphate buffer (50 mmol/L pH 7.8), 0.2 mL of deionized water and 0.1 mL of enzyme extract was placed under light with an average photon flux density of 78 μmol/(s·m2) for 20 min at 25 °C, and the absorbance of the reaction mixture was recorded at 560 nm. Reaction solution placed in the dark was used as the control. One unit of enzyme activity was defined as the amount of the enzyme that resulted in 50% inhibition of the rate of NBT reduction.
2) POD activity was determined by measuring the absorbance changes at 470 nm and 25 °C [10]. 0.1 mL of enzyme extract was added into a mixture solution of 1 mL 0.3% H2O2, 0.9 mL 0.2% guaiacol, 1 mL sodium phosphate buffer (50 mmol/L, pH 7.0) to start enzymatic reaction. One unit of POD enzyme activity was defined as the amount of the enzyme that caused an increase in absorbance at 470 nm of 0.1 per minute.
3) CAT activity was measured by monitoring the decrease of H2O2 at 240 nm for 1 min at 25 °C [16]. 0.1 mL enzyme extract was added in a mixture solution containing 1 mL 0.3% H2O2 and 1.9 mL sodium phosphate buffer (50 mmol/L, pH 7.0) to initiate the reaction. One unit of CAT activity was calculated as the amount of enzyme that caused a reduction in absorbance at 240 nm of 0.1 per minute.
4) The Sb-induced oxidative damage (membrane liquid peroxidation) was estimated by measuring the MDA concentrations [17]. The MDA content was assayed using a solution containing 3 mL of 20% (w/V) trichloroacetic acid, which included 0.5% (w/V) thiobarbituric acid and 1 mL enzyme extract. The solution was kept in boiling water bath for 20 min and then quickly cooled. After refrigeration, the homogenate was centrifuged at 5000g and 25 °C for 10 min. The absorbance of the supernatant was recorded at 450, 532 and 600 nm, respectively. The content of MDA (C1) (μmol/L) was calculated from the following formula: C1=[6.45(A532-A600)-0.56A450], where A450, A532, A600 represent the absorbance values at 532, 600 and 450 nm, respectively. The content of soluble sugar (C2) (mmol/L) was calculated by the following formula [18]: C2=11.71×A450.
2.4 Chlorophyll estimation and chlorophyll a fluorescence measurement
To determine chlorophyll and carotenoid contents in leaves, the homogenate of fresh leaves (0.2 g) was extracted for 24 h in 25 mL of 80% acetone in darkness. The contents of chlorophyll a, chlorophyll b and carotenoids in the extracts were measured at 646, 663 and 470 nm with a spectrophotometer (UV-4100, Hitachi), respectively [19].
Chl fluorescence of the fully expanded, fourth leaf from top was measured. All fluorescence measurements were carried out in darkness for 40 min to obtain equilibrium of the photosystem II (PSII) redox state. The induction curve (slow kinetics) for PSII was measured using the MINI-version of the Imaging-PAM fluorometer (Walz, Effeltrich, Germany). For fluorescence measurements, the actinic light was set at 135 μmol/(m2·s) PAR. The irradiance step was 20 s, and the length of the program was 5 min. The maximum quantum efficiency of PSII (Fv/Fm) and quantum yield of PSII electron transport (Y(II)) were calculated [20].
2.5 Statistics
Analysis of variance (ANOVA) was performed using SPSS statistical software. A p-value of <0.05 denoted significance. The Tukey test and subsequent pairwise comparisons were employed to compare significant differences (p<0.05) between the means for the treatments. All data presented are mean values of three replicate experiments.
3 Result and discussion
3.1 Growth of plants
The effects of Sb on the growth of F. tikoua were evaluated by measuring the dry mass of root, stem and leaf (Fig. 1). The dry mass of the F. tikoua leaves treated with Sb concentration higher than 30 μmol/L was significantly (p<0.05) lower than that of the untreated controls (without Sb application). But the dry mass of stem and root at all Sb concentrations was not statistically different. When heavy metal transported from matrix to roots and further moved to different parts in the shoots, the toxicity occurred, affecting various plant processes [4]. Plant organs have different sensitivities to Sb exposure because of their different biomass allocation patterns when plants were exposed to Sb stress [21]. In this study, the leaf of F. tikoua was more sensitive than root and stem under the Sb treatments.
3.2 Sb concentrations in F. tikoua
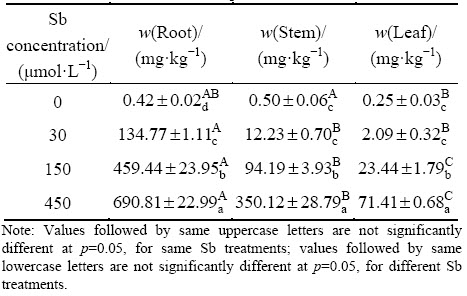
F. tikoua showed the ability to limit Sb transfer from the underground parts to the aboveground parts since Sb concentrations in the roots of F. tikoua were significantly (p<0.05) higher than that in stems and leaves for all Sb treatments (except for control) (Table 1). Sb concentration in the plants increased with increasing Sb concentration in solution. The maximum Sb concentrations in the root, stem and leaf were 690.81, 350.12 and 71.41 mg/kg respectively when the plants were exposed to 450 μmol/L Sb. According to the research of FENG et al [22] and TSCHAN et al [23], Sb accumulation in different species varies greatly [22,23]. In the present study, Sb concentration in the roots of F. tikoua was one order of magnitude higher than those in the stems and leaves, suggesting that the F. tikoua would be a plant species to tolerate Sb.
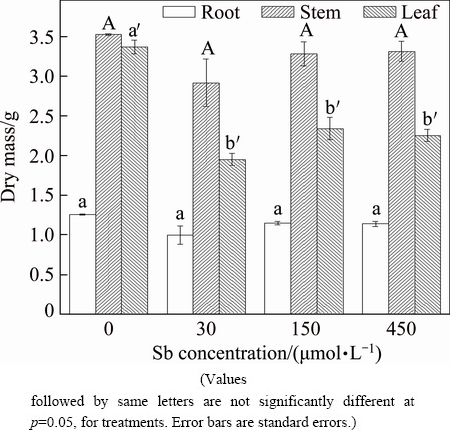
Fig. 1 Dry mass of F. tikoua under Sb treatments
Table 1 Sb content in different parts of F. tikoua
3.3 Effect of Sb on antioxidant enzyme activity
The activities of POD, SOD, and CAT responded to Sb stress with different concentrations in the different exposure periods are shown in Fig. 2. The SOD activity increased with increasing Sb concentration in matrix (Fig. 2(a)). The SOD activity in all Sb treatments is higher than that in control treatment. Especially, 450 μmol/L Sb treatment significantly resulted in increase in SOD activity during the whole experiment period as compared with control treatment. The CAT activity increased with increasing Sb concentration in matrix (Fig. 2(b)) and CAT activity in F. tikoua was improved after exposure to 150 μmol/L Sb for 7 d. All Sb treatments resulted in higher CAT activity than the control. Especially, the significant increase of CAT activity during the all exposure period was observed with 150 and 450 μmol/L Sb treatments. However, POD activity in F. tikoua has different change trends in the different exposure periods (Fig. 2(c)). In the early exposure period (24 d), POD activity significantly increased only with 450 μmol/L Sb treatment. With prolonging exposure time till 65-78 d, POD activity significantly increased with low Sb concentration treatment, such as 30 μmol/L Sb. Heavy metals exert toxic effects through the production of ROS, which have a variety of harmful effects in plant cells including lipid peroxidation [24]. To cope with ROS or alleviate their damaging effect, plants have evolved enzymatic and nonenzymatic antioxidant mechanisms. Generally, improvement of stress tolerance is related to an increase in the activity of antioxidant enzymes, and plants may experience oxidative damage due to the inability of the antioxidative enzymes to tolerate severe stress [25,26]. In the present study, to avoid oxidative damage, the complex antioxidant defense systems of plant regulated the contents or activities of antioxidants (SOD, POD and CAT) in response to Sb stress. The results indicate that Sb could significantly change the enzyme activity of F. tikoua even the concentration is as low as 30 μmol/L. Antimony could either inhibit or stimulate the activity of several antioxidant enzymes before any visible symptoms of toxicity appear [9,10]. A more efficient antioxidative system likely resulted in lower oxidative stress and reduced membrane damage.
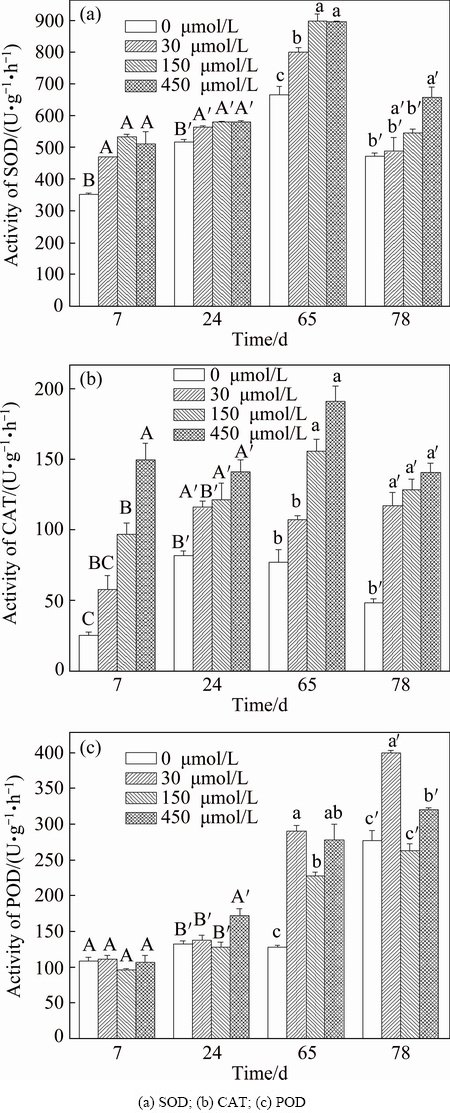
Fig. 2 Activities of antioxidant enzymes in F. tikoua under Sb treatments
3.4 Lipid peroxidation and soluble sugar content
Figure 3(a) shows MDA content of F. tikoua in all Sb treatments. In the early exposure period (7 and 24 d) the higher Sb concentration showed higher MDA content of F. tikoua than the lower Sb concentration. The highest MDA content was observed with 450 μmol/L Sb treatment at 24 d. At the end of the experiment (65 and 78 d), there was no significant difference of MDA content with 150-450 μmol/L Sb treatments and control treatment, while the highest MDA content was observed with the 30 μmol/L Sb treatment. Usually, the high level of MDA was expected because the metalloids contents were very high [25]. These suggested that F. tikoua could alleviate lipid peroxidation under different Sb levels over time. Besides, the low Sb concentration (30 μmol/L Sb) stress on F. tikoua worked later than the high one (Fig. 3). The soluble sugar was considered as the important matter to adapt the heavy metal stress [27]. In this work, soluble sugar content, except for 30 and 150 μmol/L Sb treatments at 24 d, significantly increased with increasing Sb concentration in matrix (Fig. 3(b)). The results suggest that the increase soluble sugar in the F. tikoua adapted to Sb stress was very important. These findings are in agreement with previous results reported for other heavy metals [28].
3.5 Effect of Sb on chlorophyll fluorescence
A positive effect of Sb on Chl a in F. tikoua leaves was observed at the early exposure period (7 and 24 d), and even the significant increase in Chl a content was observed with 150 and 450 μmol/L Sb treatments as compared with the control treatment (Fig. 4(a)). However, at the end of the experiment (65 and 78 d), Chl a content was recovered, and Chl a content was not significantly different among most Sb treatments except for 450 μmol/L at 78 d. The positive effect of Sb on Chl b content was observed at 7 d when Sb concentration was in the range of 30-150 μmol/L (Fig. 4(b)). But there was no significant difference of Chl b content among the most Sb treatments at 24 and 65 d, except for 30 μmol/L Sb at 24 d. At the end of the experiment (78 d), Sb stress at 450 μmol/L Sb treatment significantly decreased Chl b content as compared with control treatment. In the early exposure period (7 and 24 d), carotenoid of F. tikoua significantly increased in the 150 and 450 μmol/L Sb treatments as compared with control treatment (Fig. 4(c)). At 65 d, no significant difference of carotenoid content was observed among control, 30, 150 and 450 μmol/L Sb treatments. However, 450 μmol/L Sb treatment slightly decreased carotenoid content of F. tikoua at 78 d. Chlorophyll content is a measure of the ability of a plant to convert photosynthetic energy into biomass, or a measurement of the efficiency of a plant to produce biomass. Indeed, Chl a, Chl b, and carotenoid contents are the indication of plant as affected by stress [29]. Reduced chlorophyll content and disturbed chlorophyll synthesis were suggested to be the primary causes of diminished photosynthetic activity. Also, it was reported that the beneficial effects on plant growth in the low dose of heavy metal presence [9,30]. In this work, Chl a, Chl b and carotenoid content varied at the different Sb exposure time. For the early exposure period, Sb has a positive effect on the Chl a, Chl b and carotenoid contents, but the positive effect was not obviously improved over all the period. At the end of experiment, a significant decrease in contents of Chl a and Chl b was observed at 450 μmol/L Sb treatment. The results suggest that the change of Chl content in the F. tikoua under Sb treatment depends on the Sb concentration and the exposure time.
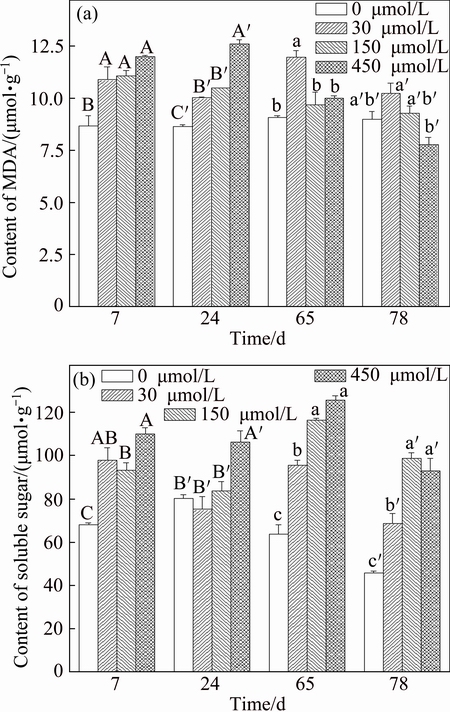
Fig. 3 Contents of MDA and soluble sugar in F. tikoua with Sb treatments
When F. tikoua was exposed to Sb treatments, the change of functional photosynthetic state was investigated by using the PAM-methodological approach and the results are shown in Fig. 5. In this study, only a considerable decline of Fv/Fm was observed with 150 and 450 μmol/L Sb treatments at 24 d, implying that F. tikoua was adapted to Sb stress on the reaction center of photosystems II (Fig. 5(a)). Y(II) provides a more realistic impression of the overall leaf photosynthetic condition when the plant is under Sb stress. In the early exposure period (7 d), Y(II) value of F. tikoua leaves in 450 μmol/L Sb treatment was higher than that with control treatment. At 24 d, a considerable increase in Y(II) was observed in 30, 150 and 450 μmol/L Sb as compared with control treatment, indicating that photosynthesis was active under Sb stress at early exposure period (Fig. 5(b)). With prolonging exposure time to 65 and 78 d, the effects of Sb stress on Y(II) were not observed because there was no significant difference of Y(II) at all treatments, indicating that photosynthesis of F. tikoua was retrieved.
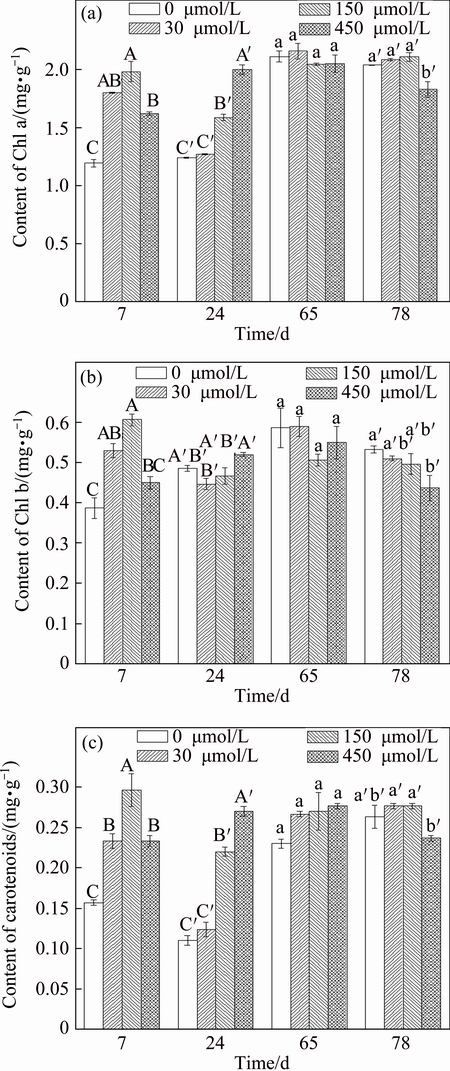
Fig. 4 Chlorophyll content of F. tikoua under Sb treatments
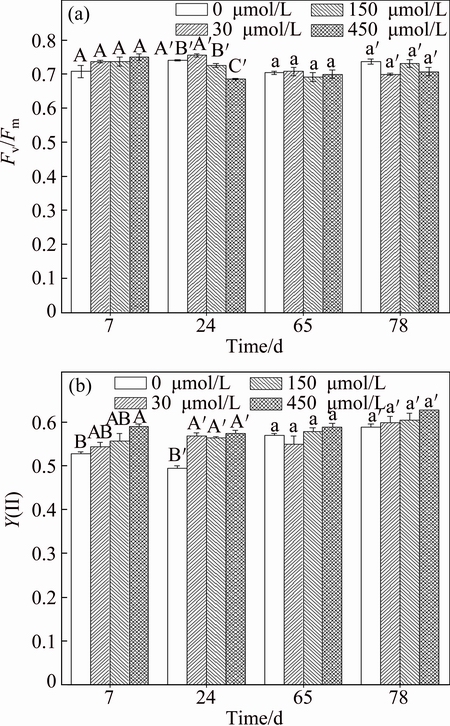
Fig. 5 Effects of Sb on fluorescence quenching parameters
Changes in PSII efficiency due to heavy metal excess were observed in different plant species and were dependent upon the time of exposure to metals and their contents in leaf tissue [30,31]. Generally, Fv/Fm is the most frequently applied Chl fluorescence ratio because it is easy and fast to determine. When plants are exposed to metal/metalloid stresses, decline in Fv/Fm indicates a disturbance or damage to the photosynthetic apparatus [30]. The present results showed that Fv/Fm and Y(II) were active under Sb stress in the early exposure period (7 d). However, there was no significant difference of Fv/Fm and Y(II) at all Sb treatments at the end of this experiment (65 and 78 d), indicating that the efficiency of the photosynthetic apparatus and the size and number of active photosynthetic centers were not inhibited or decreased in Sb concentration range of 30-450 μmol/L.
4 Conclusions
1) F. tikoua showed a certain ability to resist and tolerate toward Sb stress. The highest Sb concentration in F. tikoua tissues was found in root, followed by stem and leaf.
2) The leaf of F. tikoua was more sensitive than root and stem under Sb stress. The biomass of leaf significantly decreased in Sb treatment, but effects of Sb treatment on the biomass of root and stem were not significantly different.
3) Under Sb stress, a negative effect of ROS in F. tikoua was eliminated and alleviated by improving SOD, CAT and POD activities and increasing soluble sugar content.
4) The photosynthesis of F. tikoua was not inhibited by Sb concentration below 450 μmol/L. In the early exposure period, the photosynthesis of reaction centers of photosystems II was active under Sb stress. However, the photosynthesis of F. tikoua was retrieved in the prolonging exposure time.
References
[1] NING Z, XIAO T, XIAO E. Antimony in the soil-plant system in an Sb mining/smelting area of southwest China [J]. International Journal of Phytoremediation, 2015, 17(11): 1081-1089.
[2] MUBARAK H, CHAI L Y, MIRZA N, YANG Z H, PERVEZ A, TARIQ M, SHAHEEN S, MAHMOOD Q. Antimony(Sb) – pollution and removal techniques–critical assessment of technologies [J]. Toxicological and Environmental Chemistry, 2015, 97(10): 1-18.
[3] WEI C, DENG Q, WU F, FU Z, XU L. Arsenic, antimony, and bismuth uptake and accumulation by plants in an old antimony mine, China [J]. Biological Trace Element Research, 2011, 144(1-3): 1150-1158.
[4] PAOLI L, FIORINI E, MUNZI S, SORBO S, BASILE A, LOPPI S. Antimony toxicity in the lichen Xanthoria parietina (L.) Th. Fr [J]. Chemosphere, 2013, 93(10): 2269-2275.
[5] CHAI L Y, MUBARAK H, YANG Z H, YONG W, TANG C J, MIRZA N. Growth, photosynthesis, and defense mechanism of antimony (Sb)-contaminated Boehmeria nivea L [J]. Environmental Science & Pollution Research, 2016, 23(8): 7470-7481.
[6] MIRZA N, MUBARAK H, CHAI L Y, YANG Z H, MAHMOOD Q, YONG W, TANG C J, FAHAD S, NASIM W. Constitutional tolerance and chlorophyll fluorescence of Boehmeria nivea L in response to the antimony (Sb) and arsenic (As) co-contamination [J]. Toxicological and Environmental Chemistry, 2017, 99(2): 265-272.
[7] ZHAO X, ZHENG L, XIA X, YIN W, LEI J, SHI S, SHI X, LI H, LI Q, WEI Y. Responses and acclimation of Chinese cork oak (Quercus variabilis Bl.) to metal stress: The inducible antimony tolerance in oak trees [J]. Environmental Science and Pollution Research, 2015, 22(15): 11456-11466.
[8] ZHANG D, PAN X, MU G, WANG J. Toxic effects of antimony on photosystem II of Synechocystis sp. as probed by in vivo chlorophyll fluorescence [J]. Journal of Applied Phycology, 2010, 22(4): 479-488.
[9] PAN X, ZHANG D, CHEN X, BAO A, LI L. Antimony accumulation, growth performance, antioxidant defense system and photosynthesis of zea mays in response to antimony pollution in soil [J]. Water, Air, & Soil Pollution, 2011, 215(1-4): 517-523.
[10] FENG R, WEI C, TU S, WU F, YANG L. Antimony accumulation and antioxidative responses in four fern plants [J]. Plant & Soil, 2009, 317(1-2): 93-101.
[11] SIRISHA N, SREENIVASULU M, SANGEETA K, CHETTY C M. Antioxidant properties of Ficus species—A review [J]. International Journal of PharmTech Research, 2010, 2(4): 2174-2182.
[12] WANG Y, CHAI L, YANG Z, HUSSANI M, XIAO R, TANG C. Subcellular distribution and chemical forms of antimony in Ficus tikoua [J]. International Journal of Phytoremediation, 2017, 19(2): 97-103.
[13] JIANG Z Y, LI S Y, LI W J, GUO J M, TIAN K, HU Q F, HUANG X Z. Phenolic glycosides from Ficus tikoua and their cytotoxic activities [J]. Carbohydrate Research, 2013, 382C(18): 19-24.
[14] WANG Y, CHAI L Y, YANG Z H, MUBARAK H, TANG C. Chlorophyll fluorescence in leaves of Ficus tikoua under arsenic stress [J]. Bulletin of Environmental Contamination and Toxicology, 2016, 97(4): 576-581.
[15] TANG S, LIAO S, GUO J, SONG Z, WANG R, ZHOU X. Growth and cesium uptake responses of Phytolacca americana Linn. and Amaranthus cruentus L. grown on cesium contaminated soil to elevated CO2 or inoculation with a plant growth promoting rhizobacterium Burkholderia sp. D54, or in combination [J]. Journal of Hazardous Materials, 2011, 198: 188-197.
[16] FENG R W, WEI C Y. Antioxidative mechanisms on selenium accumulation in Pteris vittata L., a potential selenium phytoremediation plant [J]. Plant, Soil and Environment, 2012, 58(3): 105-110.
[17] QU Y, ZHOU Q, YU B. Effects of Zn2+ and niflumic acid on photosynthesis in Glycine soja and Glycine max seedlings under NaCl stress [J]. Environmental and Experimental Botany, 2009, 65(2): 304-309.
[18] CHENG Z, DONG K, GE P, BIAN Y, DONG L, DENG X, LI X, YAN Y. Identification of leaf proteins differentially accumulated between wheat cultivars distinct in their levels of drought tolerance [J]. Plos One, 2015, 10(5), DOI: 10.1371/journal.pone.0125302
[19] WELLBURN A, LICHTENTHALER H. Advances in photosynthesis research [M]. Netherlands: Springer, 1984: 9-12.
[20] KRAMER D M, JOHNSON G, KIIRATS O, EDWARDS G E. New fluorescence parameters for the determination of Q(A) redox state and excitation energy fluxes [J]. Photosynthesis Research, 2004, 79(2): 209-218.
[21] FENG R, WANG X, WEI C, TU S. The accumulation and subcellular distribution of arsenic and antimony in four fern plants [J]. International Journal of Phytoremediation, 2015, 17(4): 348-354.
[22] FENG R, WEI C, TU S, TANG S, WU F. Simultaneous hyperaccumulation of arsenic and antimony in Cretan brake fern: evidence of plant uptake and subcellular distributions [J]. Microchemical Journal, 2011, 97(1): 38-43.
[23] TSCHAN M, ROBINSON B H, SCHULIN R. Antimony in the soil–plant system—A review [J]. Environmental Chemistry, 2009, 6(2): 106-115.
[24] ZENGIN F. Biochemical and physiological effect of excess manganese (Mn) in bean (Phaseolus vulgaris L. cv. Strike) [J]. Proceedings of the National Academy of Sciences, India (Section B): Biological Sciences, 2013, 83(4): 651-657.
[25] BENHAMDI A, BENTELLIS A, RACHED O, DU L G, MECHAKRA A. Effects of antimony and arsenic on antioxidant enzyme activities of two steppic plant species in an old antimony mining area [J]. Biological Trace Element Research, 2014, 158(1): 96-104.
[26] WANG C L, LIU Y G, ZENG G M, HU X J, YING Y C, XI H U, ZHOU L, WANG Y Q, LI H Y. Mechanism of exogenous selenium alleviates cadmium induced toxicity in Bechmeria nivea ( L. ) Gaud (Ramie) [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(12): 3964-3970.
[27] ROSA M, PRADO C, PODAZZA G, INTERDONATO R, GONZ LEZ J A, HILAL M, PRADO F E. Soluble sugars: Metabolism, sensing and abiotic stress: A complex network in the life of plants [J]. Plant Signaling & Behavior, 2009, 4(5): 388-393.
[28] AZMAT R, HAIDER S, NASREEN H, AZIZ F, RIAZ M. A viable alternative mechanism in adapting the plants to heavy metal environment [J]. Pakistan Journal of Botany, 2009, 41(6): 2729-2738.
[29] CI D, JIANG D, WOLLENWEBER B, DAI T, JING Q, CAO W. Cadmium stress in wheat seedlings: Growth, cadmium accumulation and photosynthesis [J]. Acta Physiologiae Plantarum, 2010, 32(2): 365-373.
[30] DAUD M K, HE Q, LEI M, ALI B, ZHU S J. Ultrastructural, metabolic and proteomic changes in leaves of upland cotton in response to cadmium stress [J]. Chemosphere, 2015, 120: 309-320.
[31] PEREIRA W E, de SIQUEIRA D L, MART NEZ C A, PUIATTI M. Gas exchange and chlorophyll fluorescence in four citrus rootstocks under aluminium stress [J]. Journal of Plant Physiology, 2000, 157(5): 513-520.
柴立元1,2,王 勇1,杨志辉1,2,H. MUBARAK1, N. MIRZA1
1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 国家重金属污染防治工程技术研究中心,长沙 410083
摘 要:采用温室培养试验研究锑对地枇杷生理变化的影响。结果表明,当锑浓度高于30 μmol/L时,地枇杷叶片的生长明显受到抑制,而地枇杷的根和茎在所有的锑浓度下都没有明显的变化,表明地枇杷的叶片比根和茎对锑的毒性更敏感,且在根中锑浓度要高于茎和叶中的。地枇杷通过增加超氧化物歧化酶(SOD)、过氧化物酶(POD)和过氧化氢酶(CAT)活性来减少锑胁迫产生的活性氧(ROS)水平, 但在锑胁迫早期,SOD和CAT比POD的作用更明显。在锑胁迫早期,不同浓度的锑能够在一定程度上增加地枇杷叶绿素的含量,但在锑胁迫后期(实验结束时),叶绿素含量在高浓度锑(450 μmol/L)胁迫下显著降低。在整个培养阶段中,地枇杷最大光量子产量和实际光量子产量值没有显著差异,表明地枇杷在锑浓度为450 μmol/L以下时,光合作用未受明显抑制。地枇杷具有一定的锑耐受能力,可用于锑污染土壤修复。
关键词:锑;生物量;抗氧化酶活性;地枇杷;叶绿素荧光
(Edited by Xiang-qun LI)
Foundation item: Project (2012GS430203-1) supported by Science and Technology Program for Public Wellbeing, China
Corresponding author: Zhi-hui YANG; Tel: +86-731-88836804; E-mail: yangzh@csu.edu.cn
DOI: 10.1016/S1003-6326(17)60106-7

