嗜酸兼性异养菌Acidiphilium sp. DX1-1的分离、特征及其浸矿行为
来源期刊:中国有色金属学报(英文版)2013年第6期
论文作者:张燕飞 彭安安 杨 宇 柳建设 邱冠周
文章页码:1774 - 1782
关键词:Acidiphilium sp.;生物浸矿;铁闪锌矿;黄铜矿;聚羟基丁酸酯;16S rRNA
Key words:Acidiphilium sp.; bioleaching; marmatite; chalcopyrite; polyhydroxybutyrate; 16S rRNA
摘 要:研究了一株源自江西德兴铜矿矿区的中温嗜酸兼性异养菌Acidiphilium sp. DX1-1的分离、鉴定、特征及其浸矿行为。菌株Acidiphilium sp. DX1-1为短杆状革兰氏阴性菌,最适合的生长温度为30 °C,最适合的生长pH约为3.5。该菌株具有广泛的底物利用特性,可以利用有机物进行异养生长并在细胞内积累聚羟基丁酸酯,也可以利用单质硫、三价铁等无机物进行自养生长。系统发育分析表明DX1-1属于Acidiphilium属,与Acidiphilium cryptum and Acidiphilium multivorum的同源性大于99%。在铁闪锌矿生物浸出过程中,Acidiphilium sp. DX1-1 表现出极强的浸矿能力,其作用不仅仅是之前报道的作为其他自养嗜酸浸矿细菌的辅助者。在初始pH3.5时,DX1-1能够在一个月内单独地浸出铁闪锌矿中40%的锌。该浸出率高于它与A. ferrooxidans混合以及A. ferrooxidans 单独浸出铁闪锌矿(初始pH均为2.0)的浸出率。
Abstract: The isolation and characterization of a subspecies of Acidiphilium that not only acts as an enhancer of other autotrophic acidophiles in bioleaching, but also has significant leaching capacity towards marmatite was described. Acidiphilium sp. DX1?1, a Gram-negative, motile, short rod-shaped bacterium that accumulates intracellular polyhydroxybutyrate, was isolated from the Dexing copper mine area in China. It is mesophilic and acidophilic with an optimum growth at 30 °C and pH 3.5. Phylogenetic analyses identify that it is a member of genus Acidiphilium and closely related to Acidiphilium cryptum and Acidiphilium multivorum. It is mixotrophic, utilizing organic substrates and a range of inorganic substrates, such as sulfur, ferric iron and a variety of sulfide minerals. Acidiphilium sp. DX1-1 is able to bioleach 40% of the zinc content of marmatite with the initial pH 3.5 within a month, which is even higher than that of A. ferrooxidans or the mixed culture with A. ferrooxidans at even lower pH.
Trans. Nonferrous Met. Soc. China 23(2013) 1774-1782
Yan-fei ZHANG1,2, An-an PENG1,2, Yu YANG1,2, Jian-she LIU1, Guan-zhou QIU1,2
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Biohydrometallurgy of Ministry of Education, Central South University, Changsha 410083, China
Received 11 January 2013; accepted 5 June 2013
Abstract: The isolation and characterization of a subspecies of Acidiphilium that not only acts as an enhancer of other autotrophic acidophiles in bioleaching, but also has significant leaching capacity towards marmatite was described. Acidiphilium sp. DX1-1, a Gram-negative, motile, short rod-shaped bacterium that accumulates intracellular polyhydroxybutyrate, was isolated from the Dexing copper mine area in China. It is mesophilic and acidophilic with an optimum growth at 30 °C and pH 3.5. Phylogenetic analyses identify that it is a member of genus Acidiphilium and closely related to Acidiphilium cryptum and Acidiphilium multivorum. It is mixotrophic, utilizing organic substrates and a range of inorganic substrates, such as sulfur, ferric iron and a variety of sulfide minerals. Acidiphilium sp. DX1-1 is able to bioleach 40% of the zinc content of marmatite with the initial pH 3.5 within a month, which is even higher than that of A. ferrooxidans or the mixed culture with A. ferrooxidans at even lower pH.
Key words: Acidiphilium sp.; bioleaching; marmatite; chalcopyrite; polyhydroxybutyrate; 16S rRNA
1 Introduction
Bioleaching, also known as biooxidation or biomining, is the use of microorganisms to extract metals from ores and tailings. The microorganisms used for bioleaching are mainly chemoautotrophs, such as Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum spp., that gain energy by oxidization of ferrous iron (Fe2+), sulfur (S0) and reduced inorganic sulfur compounds [1]. The consequential ferric iron (Fe3+) and low pH of the leaching effluent facilitates subsequent metal oxidation and extraction [2]. These chemoautotrophs are highly sensitive to organic compounds released from themselves and by cell lysis. The dissolved organic carbon is metabolized by heterotrophic and mixotrophic microorganisms, such as Acidimicrobium, Ferroplasma, Sulfobacillus and Acidiphilium spp., that also play significant roles in the bioleaching process [3]. It would therefore be of interest to identify organisms that able to bioleach metal ores in the presence of both organic and inorganic substrates.
Acidiphilium spp. are Gram-negative, mesophilic, aerobic, acidophilic, heterotrophic, rod-shaped bacteria. The genus Acidiphilium currently contains six species: Acidiphilium acidophilum (formerly Thiobacillus acidophilus) [4-6], Acidiphilium angustum [7], Acidiphilium cryptum [8], Acidiphilium multivorum [9], Acidiphilium organovorum [10] and Acidiphilium rubrum [7]. The Acidiphilium spp. bacteria can grow on a wide range of organic substrates, and some species are able to reduce ferric iron (Fe3+) and/or oxidize elemental sulfur [8,11-13]. These species are often found in close association with the acidophilic chemoautotrophs in acidic mining environments. The type species A. cryptum was isolated from Acidithiobacillus ferrooxidans cultures [8].
In this work, we describe the isolation, characterization of Acidiphilium sp. DX1-1 and ore bioleaching by this acidophilic mixotrophic organism, which not only acts as an enhancer of other autotrophic acidophiles in bioleaching, but also has a significant leaching capacity by itself towards marmatite.
2 Experimental
2.1 Sample origin
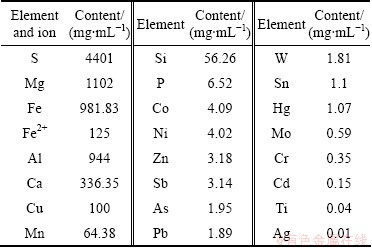
Liquid samples were collected from the Yangtao Wu reservoir in the Dexing copper mine of Jiangxi province in China, which was mainly composed of the acid mine drainage, infiltration water and rain water. The pH of collected samples was 3.0, and the water temperature was 27 °C when sampling. Chemical analysis of water sample was carried out by inductively coupled plasma-atomic emission spectroscopy (ICP-AES). Besides iron and sulfur, the most abundant metals present were magnesium and aluminium (Table 1).
Table 1 Concentrations of selected elements and ions of water sample from Yangtao Wu reservoir
2.2 Culture conditions
9K basic medium ((NH4)2SO4 3.0 g, KCl 0.1 g, KH2PO4 0.5 g, MgSO4·7H2O 0.5 g, Ca(NO3)2 0.01 g, distilled water 1000 mL) with different proportions of FeSO4·7H2O and glucose were used for flasks enrichment of the organisms [14]. 9K-glucose solid medium (9K basic medium with 1% glucose and 1.5% agar powder, pH 3.5) was used for isolation and purification. 9K-glucose liquid medium (9K basic medium with 1% glucose) was used for growing cells and determining the physiochemical characterization. 9K basic liquid medium, adjusted the pH to 3-3.5 with H2SO4, was used for energy utilization determination. All the 9K basic medium described above was sterilized by autoclaving at 121 °C for 20 min, the FeSO4·7H2O and glucose solutions were filter sterilized with a 0.22 μm cutoff filter. Inoculum was incubated under aerobic shacking conditions of 30 °C and 200 r/min.
2.3 Enrichment and isolation
Acidiphilium spp. in the water sample were enriched in the 9K basic medium supplemented with gradually increasing concentration of glucose from 0.01% to 1% and decreasing concentration of FeSO4 from 4.5% to 0 in four serial subculturing steps [6].
Isolation of glucose-grown Acidiphilium spp. colonies was performed on 9K-glucose solid medium. Eleven single colonies were picked, of which one isolate named DX1-1 was chosen for further investigation. DX1-1 was deposited in China Center for Type Culture Collection (CCTCC) under the number as CCTCC CSU 208092.
2.4 Morphology and subcellular structure observation
Morphological characteristics of the isolate were observed with an optical microscope (Olympus CX-31) after Gram staining. Surface and inner substructures of the cells harvested from the logarithmic growth phase and fixed by 4% glutaraldehyde were examined with a scanning electron microscope (SEM, JEOL JSM-6360 LV) and transmission electron microscope (TEM, JEOL JEM-1230) after cytochemical treatment and staining.
2.5 Physiochemical characterization
1 mL of log phase culture (about 1×108 cell) was inoculated into 100 mL of 9K-glucose liquid medium to determine the optimal temperature and pH for growth. Effects of the temperature and the pH on the growth of the strain were determined by direct cells counting under a microscope. Growth curves of the strain were obtained under optimal conditions and by counting the cells.
To test inorganic substrate utilization, the strain DX1-1 inoculum was inoculated in 9K basic medium supplemented with 1% (w/v) alternative inorganic substrate—FeSO4·7H2O, S0, Na2S2O3, Fe2(SO4)3, FeCl3, CuS, pyrrhotite, chalcopyrite, pyrite, marmatite and jarosite. The characteristics of the organic substrates were investigated by BIOLOG method [15]
2.6 G+C content, 16S rRNA gene sequencing and phylogenetic analysis
Cells were harvested by centrifugation and washed with TE buffer (pH 8.0). Genomic DNA was extracted by UNIQ-10 Spin Column Genomic DNA Minipreps Kit (Sangon) according to the protocol of the Kit. The G + C content of DNA was determined by China Center for Type Culture Collection (CCTCC), using HPLC as described by MESBAH et al [16].
Amplification of 16S rRNA gene of the isolate was carried out in PCR cycler (T-Gradient Thermoblock, Biometra) using universal primers 27F (5′- AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) [17]. Amplifica- tion reactions were performed in a total volume of 50 μL. The reaction mixture contained 5 μL of 10×PCR buffer, 5 μL of 20 mmol/L MgCl2, 1 μL of 10 mmol/L dNTPs mixture, 1 μL of primers 27F (5 pmol/μL) and 1492R (5 pmol/μL), respectively, 0.5 μL of Taq polymerase (NEB, 5 U/μL) and 1 μL of template DNA (about 100 ng/μL). The following thermo cycling procedure was used: an initial holding at 94 °C for 3 min, followed by 30 cycles at 94 °C for 45 s,55 °C for 1 min, and 72 °C for 1 min, then followed by an extension to the last cycle at 72 °C for 7 min. The PCR product was purified using E.Z.N.ATM Gel Extraction Kit (OMEGA). The product was integrated into pBS-T vector using the pBS-T PCR Products Clone Kit (Tiangen Biotech, Beijing). 3 μL of ligation mixture was used to transform into Escherichia coli DH5α competent cells and plated on Luria-Bertani (LB) medium containing ampicillin (100 μg/mL), 5- bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal, 200 μg/mL) and isopropyl β-D-thiogalactopyranoside (IPTG, 40 μg/mL). The white recombinant colonies were screened and sequenced on an ABI 3730 sequencer (Sunbiotech Co., Ltd, China).
The 16S rRNA gene sequence of strain DX1-1 was comparatively analyzed with the nucleic acid database of GenBank. Phylogenetic relationships of strain DX1-1 and other Acidiphilium spp. bacteria were derived by aligning sequences by Clustal X.
2.7 Extraction and identification of intracellular polymers
Intracellular polymers were extracted and recovered by SDS-EDTA method [18]. Sodium dodecyl sulfate (SDS) and EDTA were added into the cell suspension to the final concentration of 7 g/L and 10 g/L, respectively. The mixture (pH 7.0) was stirred at 50 °C for 15 min and followed by a centrifugation at 4000 r/min for 15 min. The precipitate was washed with distilled water and further purified with chloroform. The Fourier transform infrared (FTIR) spectroscopy was used to identify the purified intracellular polymers. The IR spectra of the extracted polymers and the standard PHB (Sigma-Aldrich) were recorded on a Nicolet FTIR 740 spectrometer in the range of 4000-400 cm-1 using KBr pellets.
2.8 Leaching experiment
Leaching experiments were carried out with the isolate DX1-1 and the mixture of the isolate DX1-1 and Acidithiobacillus ferrooxidans QSX-1 (accession number: CCTCC AB 206200). QXS-1 was isolated by our lab and deposited in China Center for Type Culture Collection (CCTCC). Marmatite and chalcopyrite were used for the leaching experiment separately.
2.9 Marmatite leaching experiments
The pure isolated strain DX1-1, A. ferrooxidans QSX-1 which had been propagated by cultivation on marmatite for one generation and their mixed cultures were used to leach marmatite collected from Dachang mining area of Guangxi province in China. The chemical components of the marmatite were 47.4% Zn, 16.99% Fe, 30.85% S and 4.76% others. Experiments were carried out in 250 mL flasks containing 100 mL of 9K basic medium plus 5% (w/v) of marmatite powder (below 200 mesh, <74 μm). Diluted sulfuric acid (2%) was used to adjust the pH value of the leaching solutions. Marmatite leaching by sole strain DX1-1 (initial pH 3.5), A. ferrooxidans QSX-1 (initial pH 2.0) and the mixture of the two strains (initial pH 2.0 and 3.5) during 30 d was observed and compared with control group. Approximately identical number of cells (about 1×107 cell) were used as inoculum and the experiments were conducted at 30 °C and under 200 r/min of shaking condition. 1 mL of samples were removed every 2 or 3 d for determining the concentrations of soluble Zn(II) ions by an atomic absorption spectrophotometer (Hitachi Z-8000). The lost water (sampling and evaporation) in the medium was supplemented with sterilized 9K basic medium after sampling.
2.10 Chalcopyrite leaching experiments
The chalcopyrite leaching experiments were performed with the same protocol, except the concentrations of soluble Cu(II) ions were determined every 4 d. The chalcopyrite used in the leaching experiment was collected from Daye mining area of Hubei province in China. The mineralogical composition of the chalcopyrite sample was 60.8% chalcopyrite (CuFeS2), 20.7% pyrite (FeS2), 8.4% CaCO3, 4.6% SiO2 and 5.5% others.
2.11 Nucleotide sequence accession number
The nucleotide sequences of the 16S rRNA gene reported in this study was deposited in the GenBank nucleotide sequence databases under the accession number EF556220.
3 Results
3.1 Cell morphology and ultrastructure
The isolate DX1-1 was adaptively enriched in 9K-FeSO4-glucose liquid culture, isolated and further purified from 9K-glucose solid medium. Colonies on solid medium appearing were round, ivory-white, translucent, convex, smooth and 2–3 mm in diameter after incubation at 30 °C for 3 d (Fig. 1(a)).
DX1-1 is Gram-negative, motile and short rod-shaped. Cells are (1.7±0.2) μm long and (0.7±0.05) μm wide and can form short chains (Fig. 1(b)). Transmission electron microscopy (TEM) (Fig. 1(c)) revealed a large number of granules inside the cells, accounting for approximately 90% of the cell volume. These were extracted and identified as the high molecular mass polyester polyhydroxybutyrate (PHB), which is utilized as a stored carbon source in many species of bacteria and can be harvested for using as being biodegradable thermoplastic. The FTIR spectrum of the polymer obtained from the strain DX1-1 is essentially identical to that of the standard PHB from Sigma (Fig. 2). The FTIR spectra of both PHB samples show typical ester (C=O) absorption at 1724 cm-1 and a series of intense bands located at 1000–1300 cm-1 corresponding to the stretching of the C—O bond of the ester group. The other absorption bands at 1380, 1457 and 2976 cm-1 region correspond to —CH3, —CH2 and —CH groups.
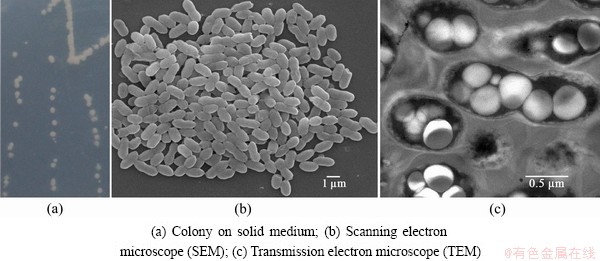
Fig. 1 Colony morphology and subcellular microstructures of strain DX1-1

Fig. 2 FTIR spectra of polymer from strain DX1-1 and standard PHB from Sigma
3.2 Optimal growth conditions
The strain DX1-1 is mesophilic and able to grow between 20 and 45 °C, with a temperature optimum of 30 °C (Fig. 3(a)). The pH range for growth is between 1.0 and 6.0 with an optimum at pH 3.5 (Fig. 3(b)). To study the growth curve of strain DX1-1, cells in the logarithmic growth phase were inoculated into flasks containing 100 mL of 9K-glucose liquid medium (pH 3.5, initial concentration 1.0×106 cell/mL) and incubated on an orbital shaker at 30 °C and 200 r/min. The growth curve indicated that the logarithmic growth phase of DX1-1 starts at about 30 h, and the stationary phase begins at about 128 h with the cell concentration reaching 1.21×109 cell/mL (Fig. 4(a)). The specific growth rate constant (μ) was calculated to be 0.09 h-1 with a mean generation time of 7.67 h.

Fig. 3 Effect of temperature (a) and pH (b) on growth of strain DX1-1
3.3 Utilization of substrates
Strain DX1-1 can grow on a wide range of inorganic substrates, including autotrophical growth by oxidizing elemental sulfur, reducing ferric iron (Fe3+) to ferrous iron (Fe2+), and utilizing jarosite and a variety of sulfide minerals (Table 2). DX1-1 grows well in the 9K basic medium supplemented with 1% elemental sulfur (Fig. 4(b)). The growth rate constant (μ) was 0.024 h-1 and the mean generation time was 28.90 h. Elemental sulfur was oxidized to the final metabolite sulfate, reducing the pH value of the medium from 3.5 to 1.0 in 17 d. DX1-1, like other sulfur oxidizing bacteria, can play an important role in bioleaching of metal sulfide ores during a process that accumulates elemental sulfur as a critical intermediate [19]. We also evaluated growth on organic substrates using the BIOLOG technique, demonstrating that DX1-1 can grow heterotrophically using a wide range of organic substrates (Table 3).

Fig. 4 Growth curves of strain DX1-1 cultured in 9K-glucose (a) and 9K-S (b) and pH change during growth in 9K-S medium (b) (Insert in (a) is the growth curve during the first 60 h)
Table 2 Inorganic substrate utilization of strain DX1-1
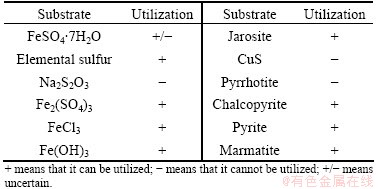
Table 3 Organic substrate utilization of strain DX1-1

3.4 Phylogenetic analysis of DX1-1
Phylogenetic analysis of the 16S ribosomal RNA (16S rRNA) gene (comprising 1450 bp, between positions 27 and 1492 in E. coli 16S rRNA gene sequence numbering) indicated that DX1-1 is a member of the alpha subclass of the proteobacteria and belongs to the genus Acidiphilium (Fig. 5). The 16S rRNA gene sequence of DX1-1 (GenBank: EF556220) shares identity of 99.72% and 99.66% to that of Acidiphilium cryptum ATCC33463 and Acidiphilium multivorum AIU301, respectively (Table 4). The G+C content of genomic DNA of strain DX1-1 is 62.3% (mole fraction), which is lower than that of all the other Acidiphilium spp. strains (Table 4).
3.5 Bioleaching studies of DX1-1
Bioleaching of marmatite and chalcopyrite by DX1-1, A. ferrooxidans QXS-1 and a mixed culture of these two strains were investigated at initial pH of 2.0 and 3.5 (Fig. 6).
In the marmatite leaching study, Acidiphilium sp. DX1-1 worked very efficiently on marmatite (Fig. 6(a)). The total extraction amount of zinc in 30 d was 9.30 g/L with the initial pH 3.5 in the leaching solution. This was about 34 times higher than the extracted amount (0.27 g/L) in the acidic media without bacteria under the same conditions. Due to the large initial inoculum, the amount of zinc extracted increased rapidly to 8.78 g/L during the first 18 d. However, the leaching rate slowed down with little contribution to the total extraction amount during the following 12 d, suggesting that the higher concentrations of the metal ions accumulated during the leaching procedure may inhibit growth. In contrast, the typical bioleaching bacterium A. ferrooxidans worked less efficiently than DX1-1 on marmatite. The total zinc extraction amount was 7.21 g/L in 30 d with the initial pH 2.0 of the leaching solution.
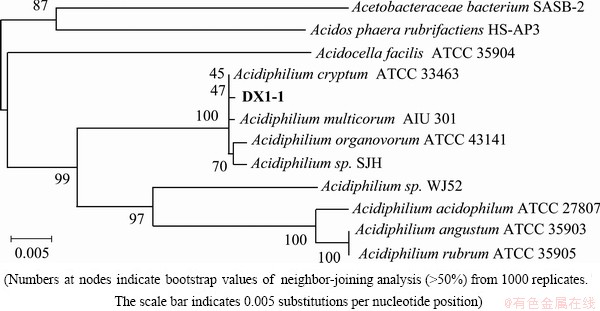
Fig. 5 Phylogenetic tree derived from 16S rRNA gene sequence of strain DX1-1
Table 4 DNA base composition of 16S rRNA homology among Acidiphilium species [8,20]
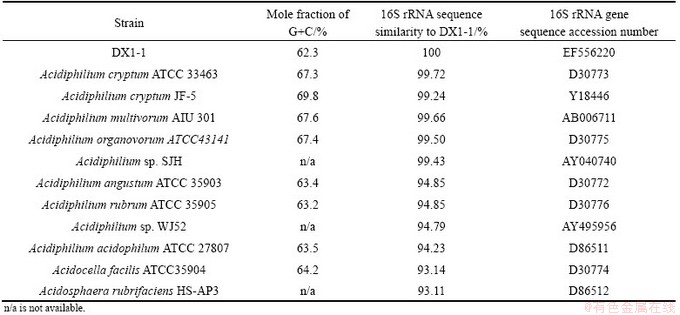

Fig. 6 Comparison of marmatite leaching (a) and chalcopyrite leaching (b) by sole or mixture of Acidiphilium sp. DX1-1 and A. ferrooxidans during 30 d flasks shaking
The zinc extraction efficiency of the mixed culture consisting of both strains (1:1) was investigated with different initial pH of the leaching solution: pH 2.0 (the optimal pH of A. ferrooxidans) and pH 3.5 (the optimal pH of Acidiphilium sp. DX1-1), respectively. The mixed culture revealed similar marmatite leaching ability to the pure culture with the same initial pH. It was deduced that Acidiphilium sp. DX1-1 was the dominant bacterium in the mixed-culture facilitated marmatite bioleaching system at its optimal pH 3.5, while A. ferrooxidans was the dominant species at pH 2.0. The mixed culture did not enhance the leaching rate of marmatite. However, the leaching capacity in the mixed culture group with the initial pH 3.5 was not inhibited after 22 d. Interactions between the two species might enhance the metal ion resistance ability of the bacteria. However, this advantage was not very obvious in the mixed culture group with an initial pH of 2.0.
In the case of chalcopyrite leaching, Acidiphilium sp. DX1-1 was not particularly effective in monoculture at pH 3.5 (Fig. 6(b)). The copper extraction amount was 0.77 g/L in 30 d, which is only slightly higher than that of the control without bacteria. However, the mixed culture of Acidiphilium sp. DX1-1 and A. ferrooxidans QXS-1 revealed a significant increase in the leaching rate of copper. The extraction amount of copper was 3.01 g/L in 30 d in the presence of mixed culture with the initial pH 2.0, which was about 1.5 times higher than that of a culture containing only A. ferrooxidans QXS-1. The leaching rate of the mixed culture with the initial pH of 3.5 was slower than that of a mixed culture with the initial pH of 2.0, but still faster than that of culture containing only A. ferrooxidans QXS-1.
4 Discussion
Our morphological and phylogenetic analyses demonstrated that strain DX1-1 is a member of genus Acidiphilium and closely related to the species A. cryptum and A. multivorum. The Acidiphilium species showed the phylogenetic heterogeneity and a relatively broad range of 16S rRNA gene sequence similarity levels which varied between 94% and 100% (Table 4) [21]. JOHNSON et al [11,22] classified all the Acidiphilium species into two groups, based on 16S rRNA gene sequence, and suggested that the bacteria in the same group are different strains of a single species rather than different species. The isolate DX1-1 belongs to the first group which includes A. cryptum, A. multivorum, A. organovorum and Acidiphilium sp. SJH. The second group includes A. acidophilum, A. organovorum and A. rubrum. The bacteria in the same group share >99% (A. organovorum and A. rubrum share 100%) 16S rRNA gene homology and have similar physiological characteristics.
Acidiphilium sp. DX1-1 can grow either heterotrophically or autotrophically and shows a relatively wide adaptability of substrate utilization. Like other Acidiphilium spp. members, DX1-1 can utilize a variety of organic substrates as carbon source. This advantage renders culturing of this bacterium easy in both liquid and solid media. This bacterium also can be incorporated into the under layer of the double-layer gel for isolating autotrophic species, such as Leptospirillum sp., because of its ability to metabolize organic compounds [23].
The intracellular PHB accumulation was also observed in other Acidiphilium species (e.g. A. angusturn ATCC 35903 [7], A. multivorum [9] and A. cryptum JF-5 [13]). PHB is the intracellular energy and carbon store that is produced by a large variety of microorganisms apparently in response to conditions of metabolic stress. It is accumulated under conditions of nitrogen or phosphorus limitation when there is an abundant carbon source in the medium, and is catabolized when other common energy or carbon sources are not available. This trait is a great advantage for DX1-1 as it allows it to be co-cultured in inorganic medium with other chemoautotrophs (such as A. ferrooxidans and Leptospirillum spp.) that are sensitive to organic compounds.
The utilization of inorganic substrates has been observed in some Acidiphilium species and strains, which renders these bacteria more likely to be considered mixotrophic. A.organovorum ATCC43141 and Acidiphilium SJH showed ferric iron reduction ability; A. rubrum is able to oxidize sulfur; A. cryptum JF-5 and A. acidophilum ATCC27807 can reduce ferric iron and oxidize sulfur. Acidiphilium sp. DX1-1 demonstrates broader inorganic substrate utilization and is able to oxidize sulfur and reduce ferric iron, as well as utilizing some sulfide minerals such as marmatite.
Prior to this work, the role of Acidiphilium spp. in bioleaching was believed to be as an enhancer of other autotrophic iron-oxidizing acidophiles by metabolizing organic compounds that inhibit growth of the iron oxidizers. However, we demonstrate here that Acidiphilium sp. DX1-1 has a significant leaching capacity towards marmatite with the initial pH 3.5, which is even higher than that of A. ferrooxidans and the mixed culture with A. ferrooxidans at even lower pH. Acidiphilium sp. DX1-1 was also able to leach chalcopyrite but to a less extent than mixed cultures containing A. ferrooxidans. Thus, Acidiphilium sp. DX1-1 acted as the enhancer of A. ferrooxidans in mixed culture during the chalcopyrite leaching process and increased the leaching capacity of A. ferrooxidans.
Overall, our work demonstrates that Acidiphilium sp. DX1-1 has properties that may render it use in metal bioleaching on an industrial scale, with the added potential utility of biopolymer synthesis during growth.
5 Conclusions
1) An acidophilic mixotrophic bioleaching bacterium Acidiphilium sp. DX1-1 was isolated. DX1-1 can grow either heterotrophically or autotrophically and shows a relatively wide adaptability of substrate utilization.
2) 9K-glucose cultured DX1-1 accumulates intracellular PHB. This trait is a great advantage for DX1-1, as it allows it to be co-cultured in inorganic medium with other chemoautotrophs that are sensitive to organic compounds.
3) DX1-1 exhibits enhanced bioleaching capacity. It not only acts as an enhancer of other autotrophic iron-oxidizing acidophiles by metabolizing organic compounds which inhibit growth of the iron oxidizers, but also has a significant leaching capacity towards marmatite.
Acknowledgements
The authors thank Dr. Joel H. WEINER and Dr. Richard A. ROTHERY for reviewing this manuscript and helpful discussion.
References
[1] JOHNSON D, HALLBERG K. The microbiology of acidic mine waters [J]. Research in Microbiology, 2003, 154(7): 466-473.
[2] ROHWERDER T, GEHRKE T, KINZLER K, SAND W. Bioleaching review part A [J]. Applied Microbiology and Biotechnology, 2003, 63(3): 239-248.
[3] JOHNSON D. Biodiversity and ecology of acidophilic microorganisms [J]. FEMS Microbiology Ecology, 1998, 27(4): 307-317.
[4] HIRAISHI A, NAGASHIMA KV, MATSUURA K, SHIMADA K, TAKAICHI S, WAKAO N, KATAYAMA Y. Phylogeny and photosynthetic features of Thiobacillus acidophilus and related acidophilic bacteria: Its transfer to the genus Acidiphilium as Acidiphilium acidophilum comb. nov. [J]. International Journal of Systematic Bacteriology, 1998, 48(4): 1389-1398.
[5] HARRISON A P. Genomic and physiological comparisons between heterotrophic thiobacilli and Acidiphilium cryptum, Thiobacillus versutus sp. nov., and Thiobacillus acidophilus nom. rev [J]. International Journal of Systematic Bacteriology, 1983, 33(2): 211-217.
[6] GUAY R, SILVER M. Thiobacillus acidophilus sp. nov.; isolation and some physiological characteristics [J]. Canadian Journal of Microbiology, 1975, 21(3): 281-288.
[7] WICHLACZ P L, UNZ R F, LANGWORTHY T A. Acidiphilium angustum sp. nov., Acidiphilium facilis sp. nov., and Acidiphilium rubrum sp. nov.: Acidophilic heterotrophic bacteria isolated from acidic coal mine drainage [J]. International Journal of Systematic Bacteriology, 1986, 36(2): 197-201.
[8] HARRISON A P. Acidiphilium cryptum gen. nov., sp. nov., heterotrophic bacterium from acidic mineral environments [J]. International Journal of Systematic Bacteriology, 1981, 31(3): 327-332.
[9] WAKAO N, NAGASAWA N, MATSUURA T, MATSUKURA H, MATSUMOTO T, HIRAISHI A, SAKURAI Y, SHIOTA H. Acidiphilium multivorum sp nov, an acidophilic chemoorganotrophic bacterium from pyritic acid mine drainage [J]. Journal of General and Applied Microbiology, 1994, 40(2): 143-159.
[10] LOBOS J H, CHISOLM T E, BOPP L H, HOLMES D S. Acidiphilium organovorum sp. nov., an acidophilic heterotroph isolated from a Thiobacillus ferrooxidans culture [J]. International Journal of Systematic Bacteriology, 1986, 36(2): 139-144.
[11] JOHNSON D B, BRIDGE T A M. Reduction of ferric iron by acidophilic heterotrophic bacteria: Evidence for constitutive and inducible enzyme systems in Acidiphilium spp [J]. Journal of Applied Microbiology, 2002, 92(2): 315-321.
[12] BRIDGE T A M, JOHNSON D B. Reductive dissolution of ferric iron minerals by Acidiphilium SJH [J]. Geomicrobiology Journal, 2000, 17(3): 193-206.
[13]  K, DORSCH T, ACKER G, STACKEBRANDT E. Microbial reduction of Fe(III) in acidic sediments: isolation of Acidiphilium cryptum JF-5 capable of coupling the reduction of Fe(III) to the oxidation of glucose [J]. Applied and Environment Microbiology, 1999, 65(8): 3633-3640.
K, DORSCH T, ACKER G, STACKEBRANDT E. Microbial reduction of Fe(III) in acidic sediments: isolation of Acidiphilium cryptum JF-5 capable of coupling the reduction of Fe(III) to the oxidation of glucose [J]. Applied and Environment Microbiology, 1999, 65(8): 3633-3640.
[14] SILVERMAN M P, LUNDGREN D G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields [J]. Journal of Bacteriology, 1959, 77(5): 642-647.
[15] GARLAND J L, MILLS A L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization [J]. Applied and Environment Microbiology, 1991, 57(8): 2351-2359.
[16] MESBAH M, PREMACHANDRAN U, WHITMAN W B. Precise measurement of the G+C content of deoxyribonucleic-acid by high-performance liquid-chromatography [J]. International Journal of Systematic Bacteriology, 1989, 39(2): 159-167.
[17] ARRIETA J M, WEINBAUER M G, HERNDL G J. Interspecific variability in sensitivity to UV radiation and subsequent recovery in selected isolates of marine bacteria [J]. Applied and Environment Microbiology, 2000, 66(4): 1468-1473.
[18] YIN J, XU Y, YU H M, ZHOU P J, SHEN Z Y. Recovery of poly-β-hydroxybutyrate from recombinant Escherichia coli by a combined biologi-chemical method [C]//TNSK I E, YONEMOTO T. Progress in Biotechnology. Amsterdami Elsevier, 2000, 16: 213-218.
[19] FOWLER T A, CRUNDWELL F K. Leaching of zinc sulfide by Thiobacillus ferrooxidans: Bacterial oxidation of the sulfur product layer increases the rate of zinc sulfide dissolution at high concentrations of ferrous ions [J]. Applied and Environment Microbiology, 1999, 65(12): 5285-5292.
[20] WAKAO N, NAGASAWA N, MATSUURA T, MATSUKURA H, MATSUMOTO T, HIRAISHI A, SAKURAI Y, SHIOTA H. Acidiphilium multivorum sp nov, an acidophilic chemoorganotrophic bacterium from pyritic acid mine drainage [J]. Journal of General and Applied Microbiology, 1994, 40(2): 143-159.
[21] KISHIMOTO N, KOSAKO Y, TANO T. Acidiphilium aminolytica sp. nov.: An acidophilic chemoorganotrophic bacterium isolated from acidic mineral environment [J]. Current Microbiology, 1993, 27(3): 131-136.
[22] JOHNSON D B, ROLFE S, HALLBERG K B, IVERSEN E. Isolation and phylogenetic characterization of acidophilic microorganisms indigenous to acidic drainage waters at an abandoned Norwegian copper mine [J]. Environmental Microbiology, 2001, 3(10): 630-637.
[23] JOHNSON D B. Selective solid media for isolating and enumerating acidophilic bacteria [J]. Journal of Microbiological Methods, 1995, 23(2): 205-218.
张燕飞1, 2,彭安安1,2,杨 宇1,2,柳建设1,邱冠周1,2
1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 中南大学 生物冶金教育部重点实验室,长沙 410083
摘 要:研究了一株源自江西德兴铜矿矿区的中温嗜酸兼性异养菌Acidiphilium sp. DX1-1的分离、鉴定、特征及其浸矿行为。菌株Acidiphilium sp. DX1-1为短杆状革兰氏阴性菌,最适合的生长温度为30 °C,最适合的生长pH约为3.5。该菌株具有广泛的底物利用特性,可以利用有机物进行异养生长并在细胞内积累聚羟基丁酸酯,也可以利用单质硫、三价铁等无机物进行自养生长。系统发育分析表明DX1-1属于Acidiphilium属,与Acidiphilium cryptum and Acidiphilium multivorum的同源性大于99%。在铁闪锌矿生物浸出过程中,Acidiphilium sp. DX1-1 表现出极强的浸矿能力,其作用不仅仅是之前报道的作为其他自养嗜酸浸矿细菌的辅助者。在初始pH3.5时,DX1-1能够在一个月内单独地浸出铁闪锌矿中40%的锌。该浸出率高于它与A. ferrooxidans混合以及A. ferrooxidans 单独浸出铁闪锌矿(初始pH均为2.0)的浸出率。
关键词:Acidiphilium sp.;生物浸矿;铁闪锌矿;黄铜矿;聚羟基丁酸酯;16S rRNA
(Edited by Hua YANG)
Foundation item: Projects (2004CB619204, 2010CB630901) supported by the National Basic Research Program of China; Project (NCET-07-0869) supported by Program of New Century Excellent Talents in Ministry of Education of China; Projects (50321402, 50374075) supported by the National Natural Science Foundation of China
Corresponding author: Yu YANG; Tel: +86-731-88877216; E-mail: csuyangyu@csu.edu.cn
DOI: 10.1016/S1003-6326(13)62660-6

