载银纳米粒子聚乙烯醇(PVA)的一锅合成及其亚甲基蓝催化还原效率
来源期刊:中国有色金属学报(英文版)2016年第10期
论文作者:P. SAGITHA K. SARADA K. MURALEEDHARAN
文章页码:2693 - 2700
关键词:载银纳米粒子聚乙烯醇;染料降解;亚甲基蓝还原;催化还原
Key words:PVA supported silver nanoparticles; dye degradation; reduction of methylene blue; catalytic reduction
摘 要:用聚乙烯醇(PVA)作为还原剂和封端剂合成稳定的银纳米粒子,采用立体稳定法将银纳米粒子掺入聚合物基体中。用紫外-可见光谱、透射电子显微镜和傅里叶变换红外光谱等手段表征掺入聚合物基体中的银纳米粒子。紫外-可见光谱结果表明合成的银纳米粒子的特征峰为426 nm,透射电子显微镜表明银纳米粒子为球形,尺寸为10~13 nm,接着用紫外光照射还原,然后采用紫外-可见光谱分析其催化性能。结果表明,合成的银纳米粒子在亚甲基蓝还原中具有良好的催化性能。
Abstract: Stable silver nanoparticles were synthesized using polyvinyl alcohol (PVA) as reducing and capping agent. The method of steric stabilization was adopted for the incorporation of silver nanoparticles in the polymer matrix. The successful incorporation of silver nanoparticles in a PVA matrix was confirmed by UV–Visible spectroscopy, transmission electron microscopy (TEM) and Fourier transform infrared (FT-IR) spectroscopy. The synthesized silver nanoparticles were characterized by a peak at 426 nm in the UV–Vis spectrum. TEM studies showed the formation of spherical shaped silver nanoparticles of 10-13 nm, following the reduction by UV irradiation. Catalytic properties were studied by means of UV-Visible spectroscopic analysis. The synthesized silver nanoparticles exhibited good catalytic properties in the reduction of methylene blue.
Trans. Nonferrous Met. Soc. China 26(2016) 2693-2700
P. SAGITHA, K. SARADA, K. MURALEEDHARAN
Department of Chemistry, University of Calicut, Malappuram-673 635, Kerala, India
Received 5 October 2015; accepted 15 April 2016
Abstract: Stable silver nanoparticles were synthesized using polyvinyl alcohol (PVA) as reducing and capping agent. The method of steric stabilization was adopted for the incorporation of silver nanoparticles in the polymer matrix. The successful incorporation of silver nanoparticles in a PVA matrix was confirmed by UV–Visible spectroscopy, transmission electron microscopy (TEM) and Fourier transform infrared (FT-IR) spectroscopy. The synthesized silver nanoparticles were characterized by a peak at 426 nm in the UV–Vis spectrum. TEM studies showed the formation of spherical shaped silver nanoparticles of 10-13 nm, following the reduction by UV irradiation. Catalytic properties were studied by means of UV-Visible spectroscopic analysis. The synthesized silver nanoparticles exhibited good catalytic properties in the reduction of methylene blue.
Key words: PVA supported silver nanoparticles; dye degradation; reduction of methylene blue; catalytic reduction
1 Introduction
From earlier time, silver nanoparticles whose structure exhibits novel physical, chemical and biological properties are elicited much interest. They are able to possess these features based on their properties such as morphology and size [1]. Silver nanoparticle-polymer composites are important class of materials having novel applications [2-8]. At a time, the polymers which are selected as support, can act as reducing agent and as stabilizing agent, thereby preventing particle growth. Appropriate functional group containing polymers can reduce the metal ions to atoms. Polymer chains are flexible and may contain variety of functional groups that are able to efficiently immobilize nanoparticles and their precursors by dispersive or van der Waals, electrostatic, hydrogen or covalent bonds. Hence, the chemical composition of the polymeric support is an important factor. In addition to these, the polymeric chain length could be tuned in order to optimize the final polymeric material architecture and thereby its performance.
Several polymers such as polyvinyl alcohol [9], polyacrylicacid [10], polyarylesters [11], poly- acrylonitrile [12] and polyvinylpyrrolidone [13], can be used as polymer support. By stabilizing with polymer matrix, the silver nanoparticles are surface modified. Hence, they can also act as capping agents. The homogenous distribution of silver nanoparticles into the polymer matrix will also increase the surface area and this makes them fit for catalytic applications. There are several methods to fabricate silver nanoparticle polymer composites [14-21]. They can be fabricated either as fibers, gels or thin films.
The stabilization of silver nanoparticles using polymer is a method of steric stabilization, which in solution is achieved by binding the polymer molecules with long alkyl chains to the particle surface. Mechanism to prevent aggregation is simple. The long alkyl chains of the polymeric moiety prevent the particles from coming close to each other. In aqueous solution, this steric stabilization is more effective. The success of immobilization of nanoparticles depends on the type of polymers used, i.e., it depends on the nature of functional group present in the polymer. In both research and technology, colloidal nanoparticles have their own importance, because of their specific properties which are not available in corresponding isolated or bulk metals.
The high reactivity of Ag nanoparticles raises difficulties in developing stable colloidal dispersions, since Ag nanoparticles rapidly undergo agglomeration. Therefore, to search the methods allowing the acquisition of nano systems with high storage stability is very important. Silver colloids stabilized by polymers in various solvents are extensively investigated [22-28]. The synthesis of mono dispersed silver nanoparticles with different size and shape has been a challenge in nanotechnology. Controlling over size and morphology of the nanoparticles is necessary during the synthesis. While preparing metal nanoparticles supported on polymer, we should ensure the homogeneous mixing of the nanoparticles in the polymer matrix. These conditions are satisfied by “in situ polymerization techniques” [29]. MBHELE et al [30] have reported a method of fabrication of silver-poly vinyl alcohol nanocomposites.
Supported silver nanoparticles have wide variety of applications in various fields such as photonics, micro electronics, sensor fabrication, optics and catalysis [31-35]. In particular, its application in catalysis is the most important and it has drawn intense attention in recent years as catalyst in organic transformation reactions [36]. Silver nanoparticles supported on organic polymers provide high surface area. The unique properties like high surface area to volume ratio, low coordination number and easy access to a large number of active sites, makes the supported silver nanoparticles a good catalyst [37]. This is because of its combined property of high reactivity and selectivity. This type of supported metal nanoparticles shows catalytic activity towards various organic reactions such as coupling, cyclo addition, reduction of dyes, and reduction of nitro phenols [23]. In comparison to bulk counterparts, specially stabilized nanoparticles have significantly high catalytic activity.
Many researchers have been working on the nanoparticles for dye degradation [38,39]. The treatment of the dye wastewater, which is harmful to the environment and the human health, is significantly important [40,41]. Efficient degradation of dyes should be done to protect the environment from severe pollution problems. Several methods are widely used to treat the dye effluents which include adsorption, biological degradation, photocatalysis, etc [40,42]. Thiazine dyes, such as methylene blue, and thionine are widely studied and used [43]. Methylene blue [3,7-bis (Dimethylamino)-phenothiazin-5-iumchloride], is a brightly coloured (ε660=105 dm3(mol/cm), blue cationic thiazine dye, with λmax values at 660, 614 and 292 nm. The reduced forms of MB, Leuco-methylene blue (λmax= 256, 314 nm) and MBH+2 (λmax=232 nm) are colourless and stable in aqueous solution [44]. It has been found that solution pH greatly influences the reduced forms of methylene blue. It seems that at low pH, methylene blue is reduced to the LMB form. At high pH, MB is demethylated and at 2<pH<7, it is reduced to the MBH+2. Some researchers had observed that the decolourization of thiazine dyes follows a first-order kinetic model [46]. MB is commonly used for colouring paper, temporary hair colorant, dyeing cottons, wools and so on. Although MB is not considered to be a very toxic dye, it can cause harmful effects, such as difficulties in breathing, vomiting, diarrhoea and nausea; it can also cause eye burns to the human and animals which may be responsible for permanent injury [45].
In recent years, JIA et al [46] have prepared porous Ag/ZnO microrods and the same was investigated in deionized water under both the UV light (15 W, 365 nm) and real sunlight irradiation. Silver modification caused the material to show significant improvement in the photocatalytic activity. 3% silver is considered the optimum concentration, which shows two times higher rate of degradation of dye than that of unmodified ZnO [46]. SURESH et al [47] synthesized novel zirconium oxide, nickel oxide and zinc oxide nanoparticles supported activated carbons (Zr-AC, Ni-AC, Zn-AC) through microwave irradiation method and the photocatalytic efficiency was verified in the degradation of textile dyeing wastewater (TDW) in UV light irradiation.
In the present study, we have developed a simple method for the preparation of poly vinyl alcohol (PVA) supported silver nanoparticles. It is an “in situ polymerization technique” in which a low temperature condition is adopted. Environmentally benign solvent water is used as the solvent, since PVA is a water soluble polymer material. The aim of the study is to find out whether it is possible to use supported silver nanoparticles as catalysts for the degradation of organic dyes. Dye degradation studies were done using methylene blue.
2 Experimental
2.1 Materials
AnalaR grade poly vinyl alcohol (PVA), silver nitrate (AgNO3), sodium hydroxide (NaOH) and sodium borohydride (NaBH4) used were all of Merck, India; assay ≥ 99.9%.
2.2 Procedure
A simple one-pot method which requires less number of chemicals and utilizes biologically benign water as the solvent was adopted for the synthesis. The synthesis route involves the following steps. To 0.1 g PVA, 50 mL of water is added, the resulting solution was heated for about few minutes at 60 °C with continuous stirring, to get a homogeneous solution of PVA in water. 1 mL of 0.2 mol/L AgNO3 and 0.2 mL of 0.3 mol/L NaOH were also added to the above solution. The colour of the solution turned yellow immediately after the addition of NaOH which indicates the formation of silver nanoparticles. The solution was stirred for 10 min and steam evaporated to remove water.
A given amount of the prepared silver nanoparticles supported on polymer were mixed with methylene blue. Volume of the mixture was adjusted to 9 mL with deionized water, 0.1 mL of NaBH4 solution was rapidly injected into it with stirring. The colour change indicates the reduction of dye.
2.3 Methods
The morphologies of the as-prepared samples were characterized by TECNAI G2 FEI FI2 TEM at an accelerating voltage of 200 kV. UV-Visible absorption spectra were measured at room temperature on a JASCO V-550 UV-Visible spectrophotometer. The IR spectrum was recorded using a JASCO FT-IR-4100 instrument.
2.4 Mechanism
There are several kinds of nanoparticles that can be embedded into a variety of solid surfaces. The mechanism of anchoring them onto the solid polymer surface is to be understood. The present method employed involves immobilization of the silver nanoparticles on poly vinyl alcohol support, by chemical reduction method.
Silver nitrate is used as the precursor material and PVA is used to act both as stabilizing and reducing agent. Silver nitrate dissociates as Ag+ ion and NO3- ion. OH group in PVA helps to reduce the silver ions to silver nanoparticles [43].
Ag+(aq)+1e→Ag(s) (1)
 (2)
(2)
This process continues until equilibrium is reached between silver nanoparticles and silver ions in the solution. The generation of silver nanoparticles can be detected by UV-Visible spectroscopy by analyzing its characteristic surface plasmon resonance band. Transmission electron microscopic techniques can also be used to find out the shape of the prepared silver nanoparticles.
The formation of silver nanoparticles can be understood from the colour change of the reaction mixture from colourless to intense yellow solution. When the reaction mixture is kept for long time, the colour changes from intense yellow to reddish brown with time, this intense colour in the visible range (for colloidal silver nanoparticles) can be attributed to the surface plasmon resonance excitations; the corresponding surface plasmon resonance bands can be observed in the range of 420-430 nm.
3 Results and discussion
3.1 Initial studies
The synthesis of silver nanoparticles depends on the selection of a suitable solvent, in which both the metal precursor and polymer are soluble. It has been found that the silver nitrate is soluble in cold water while PVA is soluble in hot water. Hence, we have used the nature friendly, environmentally benign water as the solvent for the synthesis.
The various stages of formation of silver nanoparticles are shown in Fig. 1. At the initial stage a light yellow colour was formed indicating the formation of silver nanoparticles. It has been observed that the reaction mixture is transparent and the brightness increases with time. Further increase in time changes the colour to cloudy brown. As the time proceeds, more silver ions reduced and more silver nanoparticles formed. Later it is steam evaporated to remove the solvent.

Fig. 1 Solutions of silver nanoparticles formed at different temperatures
3.2 UV-Vis spectroscopic studies of as-synthesized PVA supported silver nanoparticles
Formation of silver nanoparticles was confirmed by using UV–Vis spectral analysis. Silver nanoparticles have free electrons, which give rise to a surface plasmon resonance (SPR) absorption band due to the combined vibration of electrons of metal nanoparticles in resonance with the light wave [48]. Simple thermal treatment of metal precursor polymer mixture has generated silver nanoparticles inside the polymer. The UV absorption spectrum analysis gives a maximum absorbance peak at 426 nm (Fig. 2), which corresponds to the surface plasmon resonance peak of silver nanoparticles; the reaction was carried out at 60 °C. The characteristic surface plasmon resonance peak at 60 °C indicates that even at low temperatures it is possible to prepare Ag nanoparticles. The characteristic broad plasmon absorption band is prevalent, revealing a large size distribution of the particles.

Fig. 2 UV-Visible spectrum of Ag nanoparticles supported on PVA
3.3 Effect of time on synthesis
It has been observed that the intensity of colour of colloids increases as the time advances. This increase in intensity arises due to the formation of more and more silver nanoparticles with the increase of time. The analysis of the ultra violet spectroscopic data reveals that the intensity exponentially increases, and reaches a maximum and then decreases with the increase of reaction time. During thermal treatment, the reduction of Ag+ ions takes place because PVA acts as the reducing agent which reduces Ag+ ions to metallic silver. The increase in reduction rate can be understood from the increase in absorbance in the UV-Visible spectrum. Figure 3 shows the effect of time on the reduction of Ag+ ions in which reactions at different time intervals are considered. It has been observed that the absorbance increases with the progress of time. Initially a large increase is observed and later the effect decreases as the time proceeds. After -30 min, it was noted that the decrease in absorbance of the MB solution became constant which shows that the reduction is completed. Even after one day the absorbance remained unchanged.

Fig. 3 Effect of time on reduction of Ag+ ions
A plausible explanation is that the reaction attained equilibrium after a certain time, i.e., the reaction followed a reversible pathway.
3.4 IR spectrum studies
Figure 4 shows the characteristic IR peaks obtained of poly vinyl alcohol. The broad band peak observed at 3421 cm-1 could be assigned to stretching vibrations of —OH groups in PVA. The FTIR spectra of Ag/PVA nanocomposite show an increase in the transmittance of the band at 1375 cm-1, in comparison with band at 1420 cm-1, which indicates the decoupling between O—H and C—H vibrations due to bonding interaction between O—H and silver nanoparticles. This finding is consistent with the FTIR spectral studies reported by MBHELE et al [30]. The study suggests that the hydroxyl groups have a stronger affinity to bind with metal and facilitate the formation of a coat over the nanoparticles and favour in stabilizing the silver nanoparticles against agglomeration. Since it remains unaltered after the reaction PVA acts as a supporting material.

Fig. 4 IR spectra of PVA and PVA supported Ag nanoparticles
3.5 Transmission electron microscopic study of Ag nanoparticles supported on polymer
Nanostructures formed in polymer supports are imaged using transmission electron microscopy (Fig. 5). The shape and size of the particle can be obtained from TEM. This synthesis method and reaction conditions lead to the formation of non-agglomerated silver nanoparticles of 10-13 nm in size and having a spherical shape.
3.6 Study on catalytic properties and UV-Visible spectroscopic analysis
The UV-Visible spectrum of organic dye methylene blue (Fig. 6) gives a peak at 662 nm. Investigations on catalytic properties are done at five different concentrations of Ag nanoparticles (Fig. 7). As the concentration of silver nanocatalyst increases, the absorbance decreases, which indicates the degradation of methylene blue. The decrease of absorbance is indicative of the ability of the nanocatalyst to reduce MB. The catalytic property of Ag nanoparticle at different time intervals was also carried out.

Fig. 5 TEM image of Ag nanoparticle supported on PVA
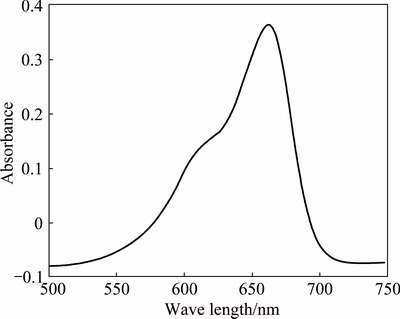
Fig. 6 UV-Visible spectrum of methylene blue
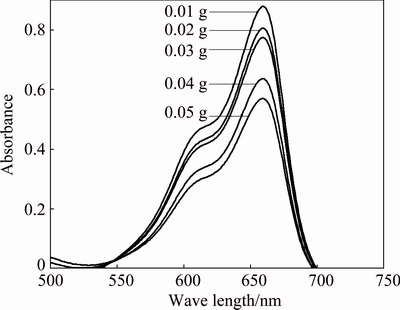
Fig. 7 UV-Visible spectra showing catalytic degradation of MB at different concentrations of Ag nanoparticles
1) 0.01 g Ag at 5 min intervals of time
As the time increases (with the same concentration of the catalyst), the catalytic activity increases. This is understood from the decrease in absorbance with the increase of time. For 0.01 g of Ag nanoparticle, the decrease in absorbance occurs slowly with a small difference in absorbance (Fig. 8).
2) 0.05 g Ag at 5 min intervals of time
The UV-Vis spectra of 0.05 g Ag at different time intervals are shown in Fig. 9. A considerable decrease in absorbance was observed when the concentration of Ag nanoparticles was increased from 0.01 to 0.05 g. The absorbance gradually decreases as the reaction time proceeds.

Fig. 8 UV-Visible spectra of 0.01 g Ag at different time

Fig. 9 UV-Visible spectra of 0.05 g Ag at different time
3.7 Kinetic study of catalytic degradation
The efficiency of catalyst can be understood from corresponding rate constant values. The rate of catalytic degradation can be calculated using ln Co/C vs time plot, where Co is the initial concentration and C is the concentration at particular time. The plot of ln Co/C against irradiation time has shown a linear relationship. The rate constants at two different concentrations (0.01 and 0.05 g of nanocatalyst) were investigated. The rate constant for each concentration was evaluated from the slope of the straight line. The rate constant increases considerably as the concentration of the catalyst increases from 0.01 to 0.05 g (Figs. 10 and 11).

Fig. 10 Plot of ln Co/C versus time for 0.01 g of nanocatalyst
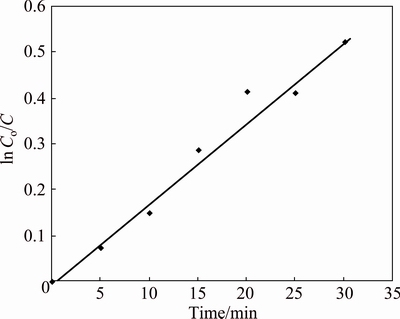
Fig. 11 Plot of ln Co/C versus time for 0.05 g of nanocatalyst
From Table 1, it is clear that the rate constant increases considerably with increase in the concentration of the nanocatalyst. This is because of the increase in the surface area of the active sites with the increase in the concentration of nanocatalysts. Hence, the rate of the reaction also increases gradually. It is observed that, as the concentration of the nanocatalyst increases, the rate constant, and thereby the rate of the reaction increases gradually.
Table 1 Rate constant of catalytic degradation of methylene blue dye in presence of Ag nanoparticles

4 Conclusions
A simple and convenient one-pot synthesis was adopted for the preparation of silver nanoparticles supported on polyvinyl alcohol polymer. The formation of silver nanoparticles was understood from the characteristic surface plasmon resonance peak obtained from the UV-Visible spectroscopic studies. The incorporation of silver nanoparticles in PVA matrix was also confirmed by IR spectroscopic studies. Morphology studies were done using transmission electron microscope. This synthesis method and reaction conditions lead to the formation of silver nanoparticles of 10-13 nm in size and spherical shape. The absorbance corresponding to the surface plasmon resonance peak at different reaction time was investigated. The increase in the absorbance with the increase of time indicates the formation of more and more Ag atoms. Catalytic studies were done on methylene blue. The chemical degradation of the dye was studied using silver nanocatalyst. Effects of different concentrations of the catalysts and also the time dependence on the catalytic activity were investigated using UV-Visible spectroscopy. The study of the absorbance with different concentrations helped to draw the conclusion that, as the concentration of the catalyst increases, the absorbance decreases gradually. This indicates that, as the concentration of the catalyst increases, corresponding increase in degradation occurs. Hence, further study using higher concentrations (>0.05 g) of silver nanoparticles are recommended. Similarly, the absorbance at different time intervals was also studied. As time proceeds, the reduction of the dye increases, this is observed from the corresponding decrease in absorbance. From the kinetic studies, the rate of the catalytic activity was calculated which showed the increase in catalytic activity with the concentration of the catalyst.
References
[1] SMITH A M, DUAN H, RHYNER M N, RUAN G, NIE S A. A systematic examination of surface coatings on the optical and chemical properties of semiconductor quantum dots [J]. Physical Chemistry Chemical Physics, 2006, 8: 3895-3903.
[2] JI Z X, ISMAIL M N, CALLAHAN D M Jr, EKO P, ZHUHUA C, GOODRICH T L, ZIEMER K S, JULIUSZ W, SACCO A Jr. The role of silver nanoparticles on silver modified titanosilicate ETS-10 in visible light photocatalysis [J]. Applied Catalysis B: Environmental, 2011, 102: 323-333.
[3] CHEN E, HAIJIA S, ZHANG W, TAN T. A novel shape-controlled synthesis of dispersed silver nanoparticles by combined bioaffinity adsorption and TiO2 photocatalysis [J]. Powder Technology, 2011, 212: 166-172.
[4] SWARNAKAR P, KANEL S R, NEPAL D, JIANG Y, JIA H, KERR L, GOLTZ M N, LEVY J, RAKOVAN J. Silver deposited titanium dioxide thin film for photocatalysis of organic compounds using natural light [J]. Solar Energy, 2013, 88: 242-249.
[5] DANGGUO G, WENG CHYE JEFFREY H, YUXIN T, QIULING T, YUEKUN L, JAMES G H, ZHONG C. Silver decorated titanate/ titania nanostructures for efficient solar driven photocatalysis [J]. Journal of Solid State Chemistry, 2012, 189: 117-122.
[6] KOSMALA A, WRIGHT R, ZHANG Q, KIRBY P. Synthesis of silver nano particles and fabrication of aqueous Ag inks for inkjet printing [J]. Materials Chemistry and Physics, 2011, 129: 1075-1080.
[7] GREER J R, STREET R A. Thermal cure effects on electrical performance of nanoparticle silver inks. [J]. Acta Materialia, 2007, 55: 6345-6349.
[8] ZHAI Dan-dan, ZHANG Tian-yu, GUO Jin-bao, FANG Xiao-hua, WEI Jie. Water-based ultraviolet curable conductive inkjet ink containing silver nano-colloids for flexible electronics [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2013, 424: 1-9.
[9] GADDY G A, KORCHEV A S, MCLAIN J L, SLATEN B L, STEIGERWALT E S, MILLS G. Light-induced formation of silver particles and clusters in cross linked PVA/PAA films [J]. Journal of Physical Chemistry B, 2004, 108: 14850-14854.
[10] WANG T C, RUBNER M F, COHEN R E. Polyelectrolyte multilayer nanoreactors for preparing silver nanoparticle composites: Controlling metal concentration and nanoparticle size [J]. Langmuir, 2002, 18: 3370-3375.
[11] RIFAI S, BREEN C A, SOLIS D J, SWAGER T M. Facile in situ silver nanoparticle formation in insulating porous polymer matrices [J]. Chemistry of Materials, 2006, 18: 21-24.
[12] ZHANG Z, HAN M. One-step preparation of size-selected and well- dispersed silver nanocrystals in polyacrylonitrile by simultaneous reduction and poly-merization [J]. Journal of Materials Chemistry, 2003, 13: 641-646.
[13] FRITZCHE W, PORWOL H, WIEGAND A, BORNMANN S,  J M. In-situ formation of Ag-containing nanoparticles in thin polymer films [J]. Nanostructured Materials, 1998, 10: 89-92.
J M. In-situ formation of Ag-containing nanoparticles in thin polymer films [J]. Nanostructured Materials, 1998, 10: 89-92.
[14] KHANNA P K, SINGH N, CHARAN S, SUBBARAO V V V S, GOKHALE R, MULIK U P. Synthesis and characterization of Ag/PVA nanocomposite by chemical reduction method [J]. Materials Chemistry and Physics, 2005, 93: 117-122.
[15] MUNRO C H, SMITH W E, GARNER M, CLARKSON J, WHITE P C. Characterization of the surface of a citrate-reduced colloid optimized for use as a substrate for surface-enhanced resonance Raman scattering [J]. Langmuir, 1995, 11: 3712-3715.
[16] HE Rong, QIAN Xue-feng, YIN Jie, ZHU Zi-kang. Preparation of polychrome silver nanoparticles indifferent solvents [J]. Journal of Materials Chemistry, 2002, 12: 3783-3786.
[17] JANA N R, GEARHEART L, MURPHY C J. Wet chemical synthesis of silver nanorods and nanowires of controllable aspect ratio [J]. Chemical Communications, 2001, 7: 617-622.
[18] CASWELL K K, BENDER C M, MURPHY C. Seedless surfactantless wet chemical synthesis of silver nanowires [J]. Nano Letters, 2003, 3: 667-672.
[19] NERSISYAN H H, LEE J H, SON H T, WON C W, MAENG D Y. A new and effective chemical reduction method for preparation of nanosized silver powder and colloid dispersion [J]. Materials Research Bulletin, 2003, 38: 949-952.
[20] PARK S, SEO D, LEE J. Preparation of Pb-free silver paste containing nanoparticles [J]. Colloids and Surfaces A, 2008, 313: 197-201.
[21] PARK K, SEO D, LEE J. Conductivity of silver paste prepared from nanoparticles [J]. Colloids and Surfaces A, 2008, 313: 351-354.
[22] ISHIZU K, FURUKAWA T, YAMADA H. Silver nanoparticles dispersed within amphiphilic star-block copolymers as templates for plasmon band materials [J]. European Polymer Journal, 2005, 41: 2853-2860.
[23] DANG G, SHI Y, FU Z, YANG W. Polymer nanoparticles with dendrimer-Ag shell and its application in catalysis [J]. Particuology, 2013, 11: 346-352.
[24] DEIVARAJ T C, LALA N L, JIM Y L. Solvent-induced shape evolution of PVP protected spherical silver nanoparticles into triangular nanoplates and nanorods [J]. Journal of Colloid and Interface Science, 2005, 289: 402-409.
[25] MACKEN A, BYRNE H J, THOMAS K V. Effects of salinity on the toxicity of ionic silver and Ag-PVP nanoparticles to Tisbe battagliai and ceramium tenuicorne [J]. Ecotoxicology and Environment Safety, 2012, 86: 101-110.
[26] MDLULI P L S, SOSIBO N M, MASHAZI P N, NYOKONG T, TSHIKHUDO R T, SKEPU A, van der LINGEN E. Selective adsorption of PVP on the surface of silver nanoparticles: A molecular dynamics study [J]. Journal of Molecular Structure, 2011, 1004: 131-137.
[27] YILMAZ E, SUZER S. Au nanoparticles in PMMA matrix: In situ synthesis and the effect of Au nanoparticles on PMMA conductivity [J]. Applied Surface Science, 2010, 256: 6630-6633.
[28] PANKAJ K R, KRISHNAMOORTHI V G S. Microwave assisted polymer stabilized synthesis of nanoparticles and its application in the degradation of environmental pollutants [J]. Materials Science and Engineering B, 2012, 177: 456-461.
[29] ALI I O. Synthesis and characterization of Ag0/PVA nanoparticles via photo- and chemical reduction methods for antibacterial study [J]. Colloids and Surfaces, 2013, 436: 922-929.
[30] MBHELE Z H, SALEMANE M G, van SITTERT C G C E, NEDELJKOVI'C J M, DJOKOVI'C V, LUYT A S. Fabrication characterization of silver-polyvinyl alcohol nanocomposites [J]. Chemistry of Materials, 2003, 15: 5019-5023.
[31] BADR Y, MAHMOUD M A. Photocatalytic degradation of methyl orange by gold silver nano-core/silica nano-shell [J]. Journal of Physics and Chemistry of Solids, 2007, 68: 413-416.
[32] COLLIER P C, SAYKALLY R J, SHIANG J J, HENRICHS S E, HEATH J R. Reversible tuning of silver quantum dot monolayers through the metal-insulator transition [J]. Science, 1997, 277: 1978-1982.
[33] SAMPAIO J F, BEVERLY K C, HEATH J R. DC transport in self-assembled 2D layers of Ag Nanoparticles [J]. Journal of Physical Chemistry B, 2001, 105: 8797-8802.
[34] LEE K S, EL-SAYED M A. Gold and silver nanoparticles in sensing and imaging: Sensitivity of plasmon response to size, shape, and metal composition [J]. Journal of Physical Chemistry B, 2006, 110: 19220-19224.
[35] SMITH G B, DELLER C A, SWIFT P D, GENTLE A, GARRETT P D, FISHER W K. Nanoparticle-doped polymer foils for use in solar control glazing [J]. Journal of Nanoparticle Research, 2002, 4: 157-160.
[36] PANDEY S, PANDEY K S, VYOM PARASHAR G K, PANDEY M C, PANDEY A C. Ag/PVA nanocomposites: Optical and thermal dimensions [J]. Journal of Materials Chemistry, 2011, 21: 17154-17159.
[37] KRSTI J, PASOJEVI J S, RADOSAVLJEVI A D ,  M, POPOVI Z K. Optical and structural properties of radiologically in situ synthesized silver nanoparticles stabilized by chitosan/poly(vinyl alcohol) blends [J]. Radiation Physics and Chemistry, 2014, 96: 158-166.
M, POPOVI Z K. Optical and structural properties of radiologically in situ synthesized silver nanoparticles stabilized by chitosan/poly(vinyl alcohol) blends [J]. Radiation Physics and Chemistry, 2014, 96: 158-166.
[38] ZENG Meng-xiong, LI You-ji, MA Ming-yuan, CHEN Wei, LI Lei-yong. Photocatalytic activity and kinetics for acid yellow degradation over surface composites of TiO2-coated activated carbon under different photocatalytic conditions [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(4): 1019-1027.
[39] DONG Shuang-shi, ZHANG Jian-bin, GAO Lin-lin, WANG Yan-long, ZHOU Dan-dan. Preparation of spherical activated carbon-supported and Er3+:YAlO3-doped TiO2 photocatalyst for methyl orange degradation under visible light [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(10): 2477-2483.
[40] ROOSTA M, GHAEDI M , SHOKRI N, DANESHFAR A, SAHRAEI R, ASGHARI A. Optimization of the combined ultrasonic assisted/adsorption method for the removal of malachite green by gold nanoparticles loaded on activated carbon: Experimental design [J]. Spectrochimica Acta (Part A): Molecular and Biomolecular Spectroscopy, 2014, 118: 55-65.
[41] GHAEDI M, ANSARI A, HABIBI M H, ASGHARI A R. Removal of malachite green from aqueous solution by zinc oxide nanoparticle loaded on activated carbon: Kinetics and isotherm study [J]. Journal of Industrial and Engineering Chemistry, 2014, 20: 17-28.
[42] ROOSTA M, GHAEDI M, DANESHFAR A, SAHRAEI R. Experimental design based response surface methodology optimization of ultrasonic assisted adsorption of safranin O by tin sulphide nanoparticle loaded on activated carbon [J]. Spectrochimica Acta (Part A): Molecular and Biomolecular Spectroscopy, 2014, 122: 223-231.
[43] ROOSTA M, GHAEDIA M, DANESHFAR A, SAHRAEIB R, ASGHARIC A. Optimization of the ultrasonic assisted removal of methylene blue by gold nanoparticles loaded on activated carbon using experimental design methodology [J]. Ultrasonics Sonochemistry, 2014, 21: 242-252.
[44] SOHRABNEZHAD S H, POURAHMAD A, RAKHSHAEE R, RADAEE A, HEIDARIANC S. Catalytic reduction of methylene blue by sulfide ions in the presence of nanoAlMCM-41 material [J]. Super Lattices and Microstructures, 2010, 47: 411-421.
[45] KAVITHA D, NAMASIVAYAM C. Experimental and kinetic studies on methylene blue adsorption by coir pith carbon [J]. Bioresource Technology, 2007, 98: 14-21.
[46] JIA Zhi-gang, PENG Kuan-kuan, LI Yan-hua, ZHU Rong-sun. Preparation and photocatalytic performance of porous ZnO microrods loaded with Ag [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(4) 873-878.
[47] SURESH1 P, JUDITH VIJAYA1 J, JOHN KENNEDY L. Photocatalytic degradation of textile dyeing wastewater through microwave synthesized Zr-AC, Ni-AC and Zn-AC [J].Transactions of Nonferrous Metals Society of China, 2015, 25(12): 4216-4225.
[48] RAJAKUMAR G, RAHUMAN A A. Acaricidal activity of aqueous extract and synthesized silver nanoparticles from Manilkara zapota against Rhipicephalus (Boophilus) microplus [J]. Research in Veterinary Science, 2012; 93: 303-309.
P. SAGITHA, K. SARADA, K. MURALEEDHARAN
Department of Chemistry, University of Calicut, Malappuram-673 635, Kerala, India
摘 要:用聚乙烯醇(PVA)作为还原剂和封端剂合成稳定的银纳米粒子,采用立体稳定法将银纳米粒子掺入聚合物基体中。用紫外-可见光谱、透射电子显微镜和傅里叶变换红外光谱等手段表征掺入聚合物基体中的银纳米粒子。紫外-可见光谱结果表明合成的银纳米粒子的特征峰为426 nm,透射电子显微镜表明银纳米粒子为球形,尺寸为10~13 nm,接着用紫外光照射还原,然后采用紫外-可见光谱分析其催化性能。结果表明,合成的银纳米粒子在亚甲基蓝还原中具有良好的催化性能。
关键词:载银纳米粒子聚乙烯醇;染料降解;亚甲基蓝还原;催化还原
(Edited by Xiang-qun LI)
Corresponding author: K. MURALEEDHARAN; Tel: +91-494-2407413; Fax: +91-494-2400269; E-mail: kmuralika@gmail.com
DOI: 10.1016/S1003-6326(16)64397-2

