Trans. Nonferrous Met. Soc. China 28(2018) 2545-2552
Leaching of cuprite through NH4OH in basic systems
A. ARACENA1, F. PEREZ1, D. CARVAJAL2,3
1. Escuela de Ingenieria Quimica, Pontificia Universidad Catolica de Valparaiso, Avenida Brasil 2162, Cod. Postal 2362854, Valparaíso, Chile;
2. Instituto de Investigacion Multidisciplinar en Ciencia y Tecnologia, Universidad de La Serena, Benavente 980, 1720170, La Serena, Chile;
3. International Organization for Dew Utilization (OPUR), 60 rue Emeriau, Paris, France
Received 9 August 2017; accepted 28 September 2018
Abstract: Cuprite is a difficult oxide to leach under acidic conditions (for the maximum extraction of 50%). In this research, the feasibility of leaching cuprite in an ammoniacal medium was studied. The working conditions addressed here were the liquid/solid ratio (120:1-400:1 mL/g), stirring speed (0-950 r/min), temperature (10-45 °C) and NH4OH concentration (0.05-0.15 mol/L). In addition, different ammoniacal reagents (NH4F and (NH4)2 SO4) were analyzed. The experiments were performed in a 2 L reactor with a heating mantle and a condenser. The most important results were that the maximum leaching rate was obtained at pH 10.5, 0.10 mol/L NH4OH, 45 °C, 4 h, 850 r/min and a liquid/solid ratio of 400:1, reaching a copper extraction rate of 82%. This result was related to the non-precipitation of copper in solution by the formation of copper tetra-amine  . The liquid/solid ratio and stirring speed were essential for increasing the cuprite leaching. The maximum leaching rate was achieved at higher temperatures; however, significant copper leaching rate occurred at temperatures near the freezing point of water (17.9% over 4 h). Increasing NH4OH concentration and decreasing particle size increased the cuprite leaching rate. The two ammoniacal reagents (NH4F and (NH4)2 SO4) had low extraction rate of copper compared with NH4OH. The kinetic model representing cuprite leaching was a chemical reaction on the surface. The order of the reaction with respect to the NH4OH concentration was 1.8, and it was inversely proportional to the radius of the ore particles. The calculated activation energy was 44.36 kJ/mol in the temperature range of 10-45 °C.
. The liquid/solid ratio and stirring speed were essential for increasing the cuprite leaching. The maximum leaching rate was achieved at higher temperatures; however, significant copper leaching rate occurred at temperatures near the freezing point of water (17.9% over 4 h). Increasing NH4OH concentration and decreasing particle size increased the cuprite leaching rate. The two ammoniacal reagents (NH4F and (NH4)2 SO4) had low extraction rate of copper compared with NH4OH. The kinetic model representing cuprite leaching was a chemical reaction on the surface. The order of the reaction with respect to the NH4OH concentration was 1.8, and it was inversely proportional to the radius of the ore particles. The calculated activation energy was 44.36 kJ/mol in the temperature range of 10-45 °C.
Key words: leaching; cuprite; ammonium hydroxide; copper tetra-amine; reaction kinetics
1 Introduction
Cuprite (Cu2O) is a copper mineral that appears in oxidized copper ores and as the product of roasting copper concentrates at high temperatures (>400 °C). This oxide can be treated using a conventional method of processing copper oxides such as the hydrometallurgical route [1], which involves a chemical process (in the presence of strong acids). However, this mineral exhibits an unusual form of dissolution. The compound dissolves upon contact with sulfuric acid, as shown in Reaction (1).
Cu2O+2H+=Cu2++Cu0+H2O (1)
As observed in the reaction, part of the cuprite dissolves; that is, 50% of the copper is extracted as cupric ions (Cu2+), and the other half is lost from the solution due to a cementation phenomenon (Cu0), making it impossible to dissolve the copper (or leach it) under acidic conditions [2]. Several authors have indicated that cuprite leaching would be produced by an electrochemical process, i.e. in the presence of an oxidant capable of capturing electrons following oxidation reduction coupling. Therefore, to treat this copper oxide (cuprite), an oxidant capable of leaching the cuprite should be used to achieve extraction rate above 50% Cu.
An attractive and alternative process is the use of aqueous ammoniacal solutions. Several studies have been performed by using this process to treat oxidized copper minerals [3-7]. However, there is a current process in which the dissolution of tenorite (CuO) in ammoniacal media with ammonium hydroxide (NH4OH) was studied [8]. In that investigation, the investigators found that tenorite can be leached in a basic system to reach a copper extraction rate of 98% in 5 h at the stoichiometric concentration of ammonium hydroxide and room temperature. And they concluded that when maintaining a pH of 10.5, the copper did not precipitate due to the interaction with the ammonium, forming copper tetra-amine ( ), which keeps the copper in solution.
), which keeps the copper in solution.
Therefore, the aim of this study was to leach cuprite (Cu2O) by using ammonium hydroxide as an alternative to sulfuric acid under different working conditions.
2 Chemistry and leaching mechanisms for cuprite
The selectivity of ammonia in basic systems has been of great interest because it can complex copper ions (I and II), and in parallel, it can precipitate impurities that do not participate in the reaction due to the alkaline medium [6-8]. For this study, the primary reactions of cuprite in an ammonium hydroxide solution are given as follows:
NH4OH= +OH- (2)
+OH- (2)
 +H2O=NH3+H3O+ (3)
+H2O=NH3+H3O+ (3)
In these two reactions, the decomposition of ammonium hydroxide (Reaction (2)) is shown, followed by the hydrolysis of the ammonium ion (Reaction (3)). Next, an oxidizing compound, such as a hydronium ion (H3O+), is generated, which would be capable of leaching the cuprite via an electrochemical process. The primary reactions with respect to cuprite are shown below
2Cu2O+2H3O+=3Cu2++2H2O+Cu(OH)2+4e (4)
Cu(OH)2+2H3O+=Cu2++4H2O (5)
The cuprite was found to leach, and cupric ions and copper hydroxide (Cu(OH)2) formed. The hydronium ion again leaches Cu(OH)2 to yield Cu2+ (Reaction (5)). Notably, there are 4 free electrons in Reaction 6. These electrons would be captured by the hydronium ion for reduction (Reaction (6)) as shown below
4H3O++4e+O2=6H2O (6)
Therefore, there would be competition for the use of hydronium ion to leach cuprite and copper hydroxide (Reactions (4) and (5)) and for its reduction (Reaction (6)).
The copper ions that are then released into the solution are complexed by ammonium to generate copper amine complexes, thus preventing the precipitation of copper in a basic medium. The formation reactions of copper amine complex are given by the following expressions:
Cu2++2NH3= (7)
(7)
 +2NH3=
+2NH3= (8)
(8)
 complex ions is thermodynamically unstable [8], and it is rapidly converted to the stable tetra-amine complex
complex ions is thermodynamically unstable [8], and it is rapidly converted to the stable tetra-amine complex  In addition, the formation of cupric ions would further increase the cuprite dissolution rates because the Cu2+ concentration would decrease to form tetra-amine, directly affecting the stoichiometric balance and causing the increased leaching rate of the solid [8]. Therefore, the global cuprite leaching reaction in a medium with NH4OH would be given by the following equation:
In addition, the formation of cupric ions would further increase the cuprite dissolution rates because the Cu2+ concentration would decrease to form tetra-amine, directly affecting the stoichiometric balance and causing the increased leaching rate of the solid [8]. Therefore, the global cuprite leaching reaction in a medium with NH4OH would be given by the following equation:
2Cu2O+8NH4OH+O2+8NH3= +4H2O+8OH- (9)
+4H2O+8OH- (9)
A phase diagram (φ-pH diagram) was constructed to predict the stability or instability of minerals in aqueous solutions. For this reason, the φ-pH diagram of the Cu2O-NH4OH-H2O system was constructed at temperatures of 10 and 45 °C. The Cu concentration was 0.018 mol/L, and the NH4OH concentration was 0.10 mol/L. The thermodynamic data used to construct the diagram were obtained from the HSC chemistry database [9]. The diagram is shown in Fig. 1.
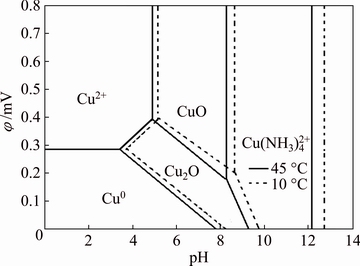
Fig. 1 Predominance diagram of Cu2O-NH4OH-H2O system at 10 and 45 °C, Cu concentration of 0.018 mol/L, and NH4OH concentration of 0.10 mol/L
As shown in Fig. 1, it is necessary to maintain pH values between 8.0 and 13.0 (for both temperatures) and potential values from 0 to 0.8 mV to ensure that  is obtained in the solution. However, it is very important that if the suggested working pH range is exceeded, the formation of tenorite (CuO) in the solution will be promoted, both at basic and acidic pH values. This effect would decrease the free cupric ions, leading to the decreasing formation of copper tetra-amines. It is also apparent that at a temperature of 10 °C, the tetra-amine remains stable, just as it does at a temperature of 45 °C. The
is obtained in the solution. However, it is very important that if the suggested working pH range is exceeded, the formation of tenorite (CuO) in the solution will be promoted, both at basic and acidic pH values. This effect would decrease the free cupric ions, leading to the decreasing formation of copper tetra-amines. It is also apparent that at a temperature of 10 °C, the tetra-amine remains stable, just as it does at a temperature of 45 °C. The  complex is not observed in the figure due to the low predominance at high potentials, so Cu(II) is stable with an excess of ammonium to form
complex is not observed in the figure due to the low predominance at high potentials, so Cu(II) is stable with an excess of ammonium to form  at these potentials. Therefore, this predominance diagram predicts that cuprite could be leached under basic pH conditions by using ammonium hydroxide over a temperature range from 10 to 45 °C.
at these potentials. Therefore, this predominance diagram predicts that cuprite could be leached under basic pH conditions by using ammonium hydroxide over a temperature range from 10 to 45 °C.
This work has the primary objective of uncovering the mechanism of dissolution for cuprite samples by using ammonium hydroxide under various experimental conditions such as different stirring speeds, temperatures, concentrations of NH4OH, particle sizes and reagents. Thus, a kinetic model could be found, which represents the dissolution of Cu2O with NH4OH with the subsequent identification of the activation energy and reaction orders.
3 Experimental
The samples obtained from Sigma Aldrich Co., consisted of fine powders (<5 μm) with a purity of 99%, and they were used for the experiments with synthetic cuprite. The pelletizing method was used for the experiments at different particle sizes, and the particles were agglomerated by the controlled pressure, thus creating spheres of 15, 25 and 38 μm.
Batch isothermal leaching experiments were performed in a 2 L glass reactor equipped with a variable mechanical stirrer, a heating element, a thermocouple, a porous liquid sample tube and a water cooled condenser to minimize the solution loss from evaporation. Figure 2 shows the experimental equipment.

Fig. 2 Experimental equipment
In a typical experiment, the reactor was loaded with 1.0 L of ammonium hydroxide leaching solution. The solution was then heated to the desired temperature, and 1.78 g of solid sample was added into the reactor. The reaction was allowed to proceed, and then, liquid samples were extracted at various time to determine the copper concentrations by atomic absorption spectroscopy (AAS). At the end of each experiment, the solution was filtered, and the residues were washed and dried, for subsequent shipment (in some cases) of X-ray diffraction analysis.
4 Results and discussion
In this chapter, we analyzed different variables that directly or indirectly affected the dissolution rate of cuprite in an ammoniacal environment. The studied variables were as follows: the solid/liquid ratio, solution stirring speed, temperature and NH4OH concentration. In addition, the effects of two ammoniacal reagents NH4F and (NH4)2SO4 were analyzed.
4.1 Effect of solution pH
In previous studies [8], the dissolution of copper oxides (in the case of tenorite, CuO) was found to be dependent on the pH of the solution, i.e. the basicity of the medium. Therefore, the zone of cuprite dissolution was studied by varying pH values (5.5, 10.5 and 14) of the solution. The working conditions were 0.10 mol/L NH4OH, 45 °C, 850 r/min, and liquid/solid ratio 400:1. The primary results are shown in Fig. 3.
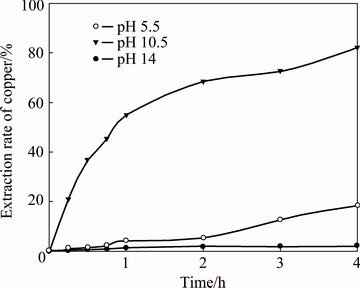
Fig. 3 Effect of solution pH on copper extraction rate
The pH of the solution strongly affects cuprite leaching. For pH values of 5.5 and 14, the copper extraction rates did not reach values over 17% after 4 h of leaching. However, for a pH value of 10.5, an approximately copper extraction rate of 82% was obtained over the same period. This result occurs primarily because of the generation of copper tetra-amine, which predominates in the pH range from 8.0 to 13.0 (Fig. 1). Outside this pH range, ionic copper would precipitate as copper oxide (CuO), thereby lowering the metal concentration in the leaching solution. Therefore, the optimum working pH for subsequent experiments was 10.5.
It can also be observed that as the leaching time increases, the copper extraction rate rapidly increases for up to approximately 2 h, while over this time, the extraction, which is translated into cuprite dissolution, becomes slower.
4.2 Effect of liquid/solid ratio
The effect of the liquid/solid ratio over a range from 120:1 to 400:1 (while maintaining a solid mass of 1.78 g) on Cu2O leaching was studied. The initial conditions were a temperature of 25 °C, a stirring speed of 800 r/min and an NH4OH concentration of 0.1 mol/L. Figure 4 summarizes the experimental data. As the solid/ liquid ratio increases, the cuprite dissolution increases because increasing the ratio assists in reactivating the diffusion medium between Cu2O and NH4OH, making the reaction more efficient. Therefore, the following leaching tests were performed at a liquid/solid ratio of 400:1.

Fig. 4 Extraction rate of copper as function of time at different solid/liquid ratios
4.3 Effect of stirring speed
To study the effect of the stirring speed, tests were performed over a range from 600 to 850 r/min at a constant temperature of 25 °C and an NH4OH concentration of 0.10 mol/L. The copper extraction rates without stirring were also reported. The results are shown in Fig. 5.
The test performed at 850 r/min reached a cuprite leaching level of approximately 58% within 1 h, reaching the maximum dissolution (82%) at the end of the test (4 h). However, when the stirring speed was reduced to 700 r/min, the copper extraction rate was reduced compared to the previous stirring (850 r/min). A copper extraction rate of 40% was reached within 1 h, and at the end of the test, the extraction rate only reached 50%. An unstirred test was also performed in which the copper recovery barely reached 4%.
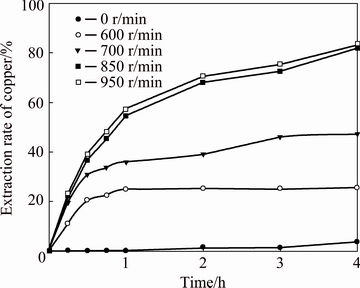
Fig. 5 Effect of stirring speed on copper extraction rate
The decrease in the leaching rate, as well as in the extraction, for lower stirring speed levels can be due to the mass transport phenomenon through an ash layer that could be formed at the time of cuprite leaching. However, this figure shows that increasing the stirring speed above 850 r/min does not increase the copper extraction rate obviously, indicating that mass transfer does not play an important role in cuprite leaching. Therefore, subsequent experiments were performed at 850 r/min.
4.4 Effect of temperature
The effect of the temperature on the copper extraction rate was analyzed. The temperature range in the study was from 10 to 45 °C. The working conditions were similar to those used previously. The relevant results are shown in Fig. 6.

Fig. 6 Effect of temperature on copper extraction rate
The temperature strongly affected the copper extraction rate as well as the leaching velocity. For a temperature of 25 °C, the extraction rate reached a value of 40.1% at 4 h, whereas at 0.5 h, a value of 19.5% was obtained. However, raising the temperature to 45 °C increased the copper extraction rate to 85.0% over the same period. In other words, when the temperature was increased by 20 °C, the copper recovery doubled.
It can also be observed that working at very low temperatures (10 °C) yields a considerable extraction rate of 17.9% over 1 h. It should be noted that the working temperatures in leaching plants in Chile reach high and low values of 46 and 8 °C, respectively. Therefore, the copper recoveries obtained in the temperature range studied here showed that this cuprite leaching alternative is very attractive.
4.5 Effect of NH4OH concentration
Figure 7 shows the copper extraction rates as a function of time in an NH4OH concentration range from 0.05 to 0.15 mol/L. The working conditions were similar to those studied so far. The figure shows that as the concentration of NH4OH increases, the copper extraction rate increases. For an NH4OH concentration of 0.08 mol/L, a 32.9% extraction rate was obtained at 1.5 h; with a concentration increase to 0.12 mol/L, an extraction rate of 95% was obtained over the same period.

Fig. 7 Effect of NH4OH concentration on copper extraction rate
It should be noted that at the lowest concentration of 0.05 mol/L NH4OH, a significant extraction rate of approximately 20% Cu was obtained. The stoichiometric NH4OH concentration (based on Reaction (9)) was 0.10 mol/L. Therefore, even at half of the stoichiometric NH4OH concentration, a significant copper extraction rate was still obtained.
4.6 Effect of different ammoniacal reagents
Different ammoniacal reagents were used to analyze the rate of copper extraction. The reagents used here were NH4F, (NH4)2SO4 and NH4OH. The same working conditions as those used in previously studies were used here. The concentration of the reagent was kept constant at 0.10 mol/L as a function of variable  . The results are shown in Fig. 8.
. The results are shown in Fig. 8.

Fig. 8 Effect of different ammoniacal reagents on copper extraction rate
Both (NH4)2SO4 and NH4F yielded copper extraction rates of only 10% within 1 h. However, NH4OH yielded a copper extraction rate of 55% over the same period, predominating over the other two reactants. At 4 h (the end of the experiment), the amount of copper extracted with NH4OH exceeds that with NH4F and (NH4)2SO4 by almost 4-fold. Therefore, these results demonstrate that NH4OH is more effective for the leaching of cuprite compared with other ammoniacal reagents (NH4F and (NH4)2SO4).
4.7 Effect of average particle size
The effect of the average particle size on the cuprite leaching rate was studied. Four particle sizes were 5, 15, 25 and 38 μm. The copper extraction rate curves are shown in Fig. 9. It is clear that increasing the particle size causes a decrease in the copper extraction rate. Thus, at a particle size of 15 μm, a copper extraction rate nearly 49.3% was obtained at 1.5 h. At a particle size of 38 μm, the copper extraction rate reached 15.2% over the same period. The decrease in the cuprite dissolution rate for larger particles may be due to the decrease in the interfacial area of the reaction.

Fig. 9 Effect of average particle size of ore samples on copper extraction rate
4.8 Kinetic study
Figure 6 shows that the temperature effect was significant on the copper extraction from cuprite. This temperature behavior suggests that the process is controlled by a chemical reaction on the surface of the mineral. Therefore, assuming that the reaction is controlled by chemical reaction, the rate equation for a recessive nucleus model by chemical control on the surface of the ore of radius ro with a constant concentration of reagent (NH4OH) can be written as follows [10]:
 (10)
(10)
where X is the reacted cuprite fraction, t is the reaction time, and kapp is the apparent rate constant, which is given by the following general expression:
 (11)
(11)
where kint is the intrinsic rate constant, [NH4OH] is the NH4OH concentration, b is a stoichiometric constant, n is the order of the reaction with respect to the NH4OH concentration, and ro is the initial radius of the ore particle.
Figure 10 shows a graph of 1-(1-X)1/3 as a function of time for the experimental data obtained from Fig. 6 over a temperature range from 10 to 45 °C for cuprite samples of 5 μm. This figure shows a good linear fit to the kinetic data with regression coefficients, expressed as R2, close to 0.96 for the entire temperature range, indicating the applicability of Eq. (10). The values of the apparent kinetic constants at various temperatures were obtained from the slopes of the straight lines and are shown in Table 1.
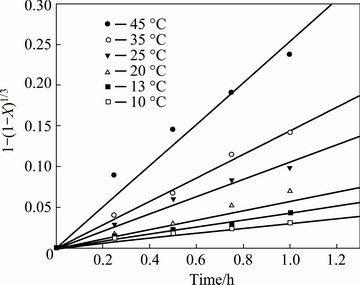
Fig. 10 Dissolution kinetics of cuprite with average particle size of 5 μm in NH4OH-H2O system under leaching conditions given in Fig. 6
Table 1 Apparent rate constants as function of temperature for cuprite leaching with NH4OH

However, the order of reaction n was calculated from the kinetic data on the effects of the NH4OH concentration in the solution (Fig. 7). Figure 11 shows the experimental data for various NH4OH concentrations plotted according to Eq. (10). The kapp values were used to draw a graph of ln kapp versus ln[NH4OH], as shown in Fig. 12. This figure shows a linear relationship, with R2 value equal to 0.92 and a slope indicating that the reaction order with respect to the NH4OH concentration in the solution is 1.8.

Fig. 11 Leaching kinetics of cuprite samples with average particle size of 5 μm at various NH4OH concentrations under leaching conditions used in Fig. 7
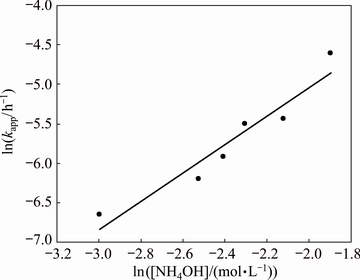
Fig. 12 Reaction order with respect to NH4OH concentration in solution
For kinetics controlled by chemical reaction, k should vary linearly with the inverse of the initial particle radius, as indicated in Eq. (11). To verify this dependence, data concerning particle sizes (Fig. 9) were fit according to Eq. (11), and the results are shown in Fig. 13.
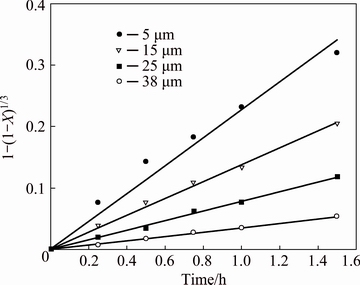
Fig. 13 Dissolution kinetics of cuprite samples with four different average particle sizes under leaching conditions used in Fig. 9
A good correlation (R2 close to 0.99) can be observed in Fig. 13, which validates Eq. (10) for the surface chemical reaction model. The kapp values obtained from Fig. 13 are plotted in Fig. 14 as a function of the inverse of the initial particle radius (Eq. (11)). The adequate linear dependence of the data shown in Fig. 14 (R2>0.89) supports the kinetic model used here.

Fig. 14 Dependence of reaction rate constant on inverse of initial particle size for cuprite leaching
Notably, the intrinsic leaching rate constant kint at different temperatures can be calculated using the values of apparent kinetic constants obtained from Fig. 10. The value of n is 1.8, and the particle radius is 2.5 μm. The value of b equals 1/4 according to the stoichiometry of Reaction (9). Table 2 shows the values of the intrinsic rate constant in the range of temperatures used in this study.
Table 2 Intrinsic rate constants as function of temperature for cuprite leaching with NH4OH

The calculated values for the intrinsic rate constants, kint, were used to draw the Arrhenius graph shown in Fig. 15. This figure shows a good linear fit (R2>0.97) for the apparent kinetic constants of each temperature. The calculated activation energy was 44.36 kJ/mol in the temperature range of 10-45 °C, which is typical for a chemical reaction on a cuprite surface.

Fig. 15 Arrhenius plot for cuprite leaching in NH4OH-H2O medium
Therefore, the apparent intrinsic kinetic constant could be represented by the following expression:
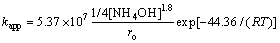 (12)
(12)
where R is the mole gas constant equal to 8.314 J/(mol·K), ro is in μm, [NH4OH] is in mol/L, t is in h, T is in K and kint =5.37×107 μm·mol/(L·h).
5 Conclusions
1) The mechanism of cuprite leaching in the presence of ammonium hydroxide occurs through the production of copper tetra-amine without the generation of any intermediate compounds.
2) The studied variables, such as the stirring speed, temperature, ammonium hydroxide concentration, particle size and solid/liquid ratio directly affect the cuprite leaching rate.
3) The kinetic equation representing the leaching of cuprite indicates chemical control on the surface of the particle, obtaining a calculated activation energy of 44.36 kJ/mol and a reaction order with respect to the NH4OH concentration of 1.8.
4) The leaching rate of cuprite is shown to be inversely proportional to the initial particle size.
References
[1] HABASHI F. Principles of extractive metallurgy: Hydrometallurgy (Volume 2) [M]. New York: Gordon & Breach, 1980.
[2] DOMIC E. Hidrometalurgia, fundamentos, procesos y aplicaciones [M]. Santiago: Editorial Null, 2001. (in Spanish)
[3] BINGOL D, CANBAZOGLU M, AYDOGAN S. Dissolution kinetics of malachite in ammonia/ammonium carbonate leaching [J]. Hydrometallurgy, 2005, 76: 55-62.
[4] EKMEKYAPAR A, OYA R, KUNKUL A. Dissolution kinetics of an oxidized copper ore in ammonium chloride solution [J]. Chemical and Biochemical Engineering Quarterly, 2003, 17: 261-266.
[5] LIU W, TANG M T, TANG C B, HE J, YANG S H, YANG J G. Dissolution kinetics of low grade complex copper ore in ammonia-ammonium chloride solution [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 910-917.
[6] BINGOL D, CANBAZOGLU M D. Dissolution kinetics of malachite in sulphuric acid [J]. Hydrometallurgy, 2004, 72: 154-165.
[7] EKMEKYAPAR A, AKTAS E, KUNKUL A, DMIRKIRAN N. Investigation of leaching kinetics of copper from malachite ore in ammonium nitrate solutions [J]. Metallurgical and Materials Transactions B, 2012, 43: 764-772.
[8] ARACENA A, VIVAR Y, JEREZ O, VASQUEZ D. Kinetics of dissolution of tenorite in ammonium media [J]. Mineral Processing & Extractive Metallurgy Review, 2015, 36: 317-323.
[9] ROINE A. HSC Chemistry 6.0 [M]. Pori, Finlandia: OutoKumpu Research Py, 1999.
[10] SHON H Y, WADSWORTH M E. Rate processes of extractive metallurgy [M]. New York: Plenum Press, 1979.
在碱性体系中用NH4OH浸出赤铜矿
A. ARACENA1, F. PEREZ1, D. CARVAJAL2,3
1. Escuela de Ingenieria Quimica, Pontificia Universidad Catolica de Valparaiso, Avenida Brasil 2162, Cod. Postal 2362854, Valparaiso, Chile;
2. Instituto de Investigacion Multidisciplinar en Ciencia y Tecnologia, Universidad de La Serena, Benavente 980, 1720170, La Serena, Chile;
3. International Organization for Dew Utilization (OPUR), 60 rue Emeriau, Paris, France
摘 要:在酸性条件下,赤铜矿是一种难浸出的氧化物,最大浸出率仅为50%。本文作者研究在氨性介质中浸出赤铜矿的可行性。所研究的实验条件为液固比(120:1~400:1 mL/g)、溶液搅拌速度(0~950 r/min)、温度(10~45 °C)和NH4OH浓度(0.05~0.15 mol/L)。此外,还对不同的氨性试剂(NH4F和(NH4)2SO4)的作用进行分析。实验是在带有加热套和冷凝器的2 L反应器中进行的。最重要的结果是,在pH 10.5、NH4OH浓度 0.10 mol/L、浸出温度 45 °C、浸出时间4 h、搅拌速度850 r/min、液固比400:1的条件下,达到82%的最大浸出率。这一结果与溶液中四氨合铜离子( )的形成使得铜不会生成沉淀有关。液固比和溶液搅拌速度是提高赤铜矿浸出率的关键参数。虽然最大浸出率是在较高温度下实现的,但在接近水冰点的温度下也发生了显著的浸出,4 h浸出率达17.9%。随着NH4OH浓度的增大和粒径的减小,Cu2O的浸出率增大。本文研究两种氨性试剂(NH4F和(NH4)2SO4),与氢氧化铵相比,它们对铜的浸出率较低。Cu2O浸出的动力学符合表面化学反应模型,相对于氢氧化铵浓度的反应级数为1.8,表观反应速率常数与颗粒半径成反比。在10~45 °C温度范围内,计算得到的活化能为44.36 kJ/mol。
)的形成使得铜不会生成沉淀有关。液固比和溶液搅拌速度是提高赤铜矿浸出率的关键参数。虽然最大浸出率是在较高温度下实现的,但在接近水冰点的温度下也发生了显著的浸出,4 h浸出率达17.9%。随着NH4OH浓度的增大和粒径的减小,Cu2O的浸出率增大。本文研究两种氨性试剂(NH4F和(NH4)2SO4),与氢氧化铵相比,它们对铜的浸出率较低。Cu2O浸出的动力学符合表面化学反应模型,相对于氢氧化铵浓度的反应级数为1.8,表观反应速率常数与颗粒半径成反比。在10~45 °C温度范围内,计算得到的活化能为44.36 kJ/mol。
关键词:浸出;赤铜矿;氢氧化铵;四氨合铜;反应动力学
(Edited by Wei-ping CHEN)
Corresponding author: A. ARACENA; E-mail: alvaro.aracena@pucv.cl
DOI: 10.1016/S1003-6326(18)64901-5