
Microstructure and properties of Al-Cu-Mg-Ag alloy exposed at 200 ℃ with and without stress
XIA Qing-kun(夏卿坤)1,2,3, LIU Zhi-yi(刘志义)1,2, LI Yun-tao(李云涛)1,2
1. Key Laboratory of Nonferrous Metal Materials Science and Engineering, Ministry of Education,
Central South University, Changsha 410083, China;
2. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
3. Department of Mechanical and Electrical Engineering, Changsha University, Changsha 410003, China
Received 28 August 2007; accepted 8 April 2008
Abstract: The effect of stress on the microstructure and properties of an Al-Cu-Mg-Ag alloy under-aged at 165 ℃ for 2 h during thermal exposure at 200 ℃ was investigated. The tensile experimental results show that the remained tensile strength of both specimens at room temperature after being exposed at 200 ℃ with and without applying stress rises firstly, and then drops with the increasing of exposure time. The peak value of the remained strength reaches 439 MPa for non-stress-exposure for 10 h, and 454 MPa after being exposed with stress loaded for 20 h at 220 MPa. The elongation change is similar to that of strength. After being exposed for 100 h, specimen exposed at 220 MPa still remains a tensile strength of 401 MPa, larger than that exposed without applying stress. TEM shows that the microstructure of under-aged alloy is dominated by Ω phase mainly and a little θ′ phase. The θ′ and Ω phases are believed competitive with increasing exposure time. The width of precipitation free zone(PFZ) increases and the granular second phase precipitates at grain-boundary correspondingly. It is shown that the mechanical properties of alloy decrease slightly and present good thermal stability after thermal exposure at 200 ℃ and 220 MPa for 100 h.
Key words: Al-Cu-Mg-Ag alloy; thermal exposure; stress; microstructure; mechanical properties
1 Introduction
Al-Cu-Mg alloys are important structural materials in aerial industry and their working temperature is be low 100 ℃. When alloys are exposed at temperature above 100 ℃, the coarsening precipitates decrease the mechanical properties[1]. Trace addition of Ag (0.1%, mole fraction) greatly changed the precipitation process of artificially aged Al-Cu-Mg alloys. Ag added in Al-Cu-Mg alloys with high Cu-to-Mg ratios promoted precipitation of a dispersed metastable Ω phase that was nucleated on the {111}α planes of matrix and grew up into a piece of thin hexagonal-shaped plate[2-4]. The Ω phase with high ability of precipitation hardening, high strength and high thermal stability at high temperature had wide application in the aviation industry and got the attention of materials researchers[5].
Recently, there is increasing interests in Al-Cu-Mg-Ag alloys research worldwide[6-9]. Some researchers[10-13] studied the creep property and showed an improved creep resistance of Al-Cu-Mg-Ag alloys on under-aged temper condition. LUMLEY et al[10-11] thought the excellent creep resistance of under-aged alloy resulted from “free solute” in solid solution. These solutes hindered the motion of dislocation in the course of creep. Some researchers[14-16] reported the effect of stress on the nucleation and coarsening of Ω precipitate in Al-Cu-Mg-Ag alloys. Results showed that stress less than yield strength changed the precipitation process during aging treatment.
SKROTZKI et al[14] studied the effect of an externally applied tensile stress on nucleation and growth of Ω and θ′ precipitates in Al-Cu-Mg-Ag alloys at 160 ℃ and found that for solution treatment samples aged under critical stress between 120 MPa and 140 MPa, Ω precipitates occur preferentially parallel to the stress axis. However, little study was reported on the stress effect at elevated exposing temperature and elevated stress in Al-Cu-Mg-Ag alloys.
In this work, the effect of 220 MPa stress on the microstructure and mechanical properties of an under-aged Al-4.72Cu-0.45Mg-0.54Ag-0.17Zr alloy during thermal exposure at 200 ℃ was investigated.
2 Experimental
The experimental alloy was prepared by pure Al(99.97%), Mg(99.9%), Ag(99. 9%) and master alloys such as Al-49.30%Cu, Al-10.0%Mn, Al-4.0%Zr, which were melted and refined in a graphite crucible under the protection of flux and then cast in iron mould. The casting alloy was homogenously treated at 420 ℃ for 6 h and 515 ℃ for 6 h, and then the alloy was rolled into 1 mm-thick sheet at 470 ℃. The strips were solid solution treated(SST) at 515 ℃ for 6 h, water quenched(WQ) and aged at 165 ℃ for 2 h. The thermal exposure at 200 ℃ for under-aged samples was carried out in constant temperature drying oven, and thermal exposure at 200 ℃ with loaded stress of 220 MPa for the alloy was carried out on creep exposure experimental machine, and the exposure time was 0, 10, 20, 50, 80, 100 h respectively. The mechanical properties at room temperature were performed on CSS-44100 type electronic tensile testing machine. Specimens for transmission electron microscopy(TEM) were punched mechanically from the strips and twin-jet electrolytically polished in a solution of 33% nitric acid and 67% methanol(volume fraction). The polishing conditions were -20 ℃, and a voltage of 12-15 V. The TEM investigation was performed on Philips TECNAL-G2 20 microscope, and its accelerating voltage was 200 kV. The KYKY-2800 scanning electron microscopy(SEM) was used to reveal the morphology of fracture.
3 Results and discussion
3.1 Mechanical properties
The change of mechanical properties of Al-Cu-Mg-Ag alloys aged at 165 ℃ for 2 h and after thermal exposure at 200 ℃ in different time is presented in Fig.1. Fig.1 showed that the remained strength of the alloy increased firstly, and then decreased subsequently with the increasing of thermal exposure time. The elongation of the alloy decreased with time and the change of elongation went gradually to smooth after thermal exposure for 20 h. The maximum surplus strength of alloys appeared at thermal exposure for 10 h. Its surplus intensities σb and σ0.2 were 439.12 MPa and 414.04 MPa, respectively, and corresponding elongation was 9.72%. After thermal exposure for 100 h, its remained strengths σb and σ0.2 were 386.95 MPa and 349.92 MPa, respectively, and corresponding elongation was 8.32%. The mechanical properties of the thermal exposure alloy decreased slightly in comparison with under-aged alloy. The process of thermal exposure was essentially re-aging. Since the microstructure of under-aged alloy was unstable, the thermal process after aging actually increased the aging process of the alloy, while the increasing of thermal exposure temperature accelerated this process[17]. During the process of under-aging treatment, the promoting aging effect caused by strong interaction between Mg and Ag not only rapidly made up the loss of strength of as-quenched alloy, but also greatly speeded up the aging process and made the strength of the alloy rise rapidly. The strength peak (σb and σ0.2) after thermal exposure for 10 h is shown in Fig 1.
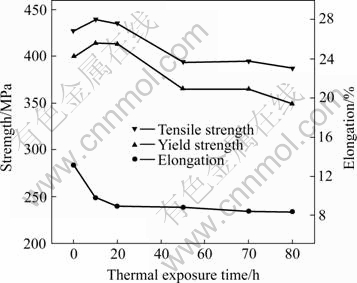
Fig.1 Curves of mechanical properties of alloy under thermal exposure
The change of mechanical properties of Al-Cu-Mg-Ag alloys aged at 165 ℃ for 2 h and after thermal exposure at 200 ℃ and 220 MPa in different time is presented in Fig.2. The surplus strength of alloy first increased, and subsequently decreased with the increasing of thermal exposure time. The strength peak appeared after thermal exposure for 20 h. Its surplus strength σb was 454 MPa and σ0.2 was 442 MPa. The strength of the alloy slowly decreased with the increasing of thermal exposure time.
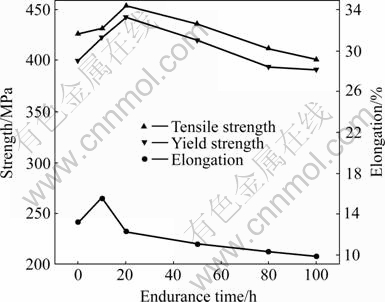
Fig.2 Curves of mechanical properties of alloy under thermal exposure at 200 ℃ and 220 MPa
The change of elongation was similar to that of strength. It was worth paying attention to the phenomenon that the elongation peak appeared after thermal exposure for 10 h, and corresponding elongation was 15.2%. After thermal exposure for 100 h, its surplus strength σb was 401 MPa and σ0.2 was 391 MPa, and corresponding elongation was 9.7%. The mechanical properties of the alloy did not lower obviously, thus indicating the excellent thermal stability.
The fractographs of Al-Cu-Mg-Ag alloys aged at 165 ℃ for 2 h and subsequently after thermal exposure at 200 ℃ and 220 MPa in different time are shown in Fig.3. The tensile fracture at room temperature presented typical ductility crack feature belonging to inner-crystal rupture and deep dimples were visible. This indicated that the ductility of the under-aged alloy was good. The mode of crack shown in Fig.3(b) was still inner-crystal rupture.
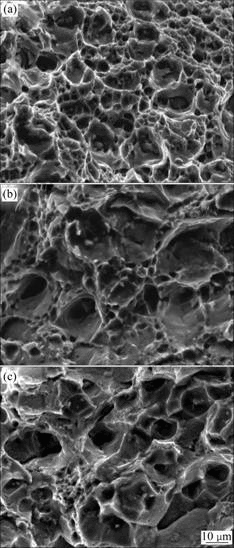
Fig.3 Fracture surface images of alloy under different thermal exposure conditions: (a) Original state; (b) Exposed for 20 h; (c) Exposed for100 h
The local plastic deformation area of fracture of the alloy was not significant compared with that of initial state alloy. There was intergranular cracking in some place of fracture and dimples were shallow. No dimple area on the crack area appeared around grain boundary, which showed the feature of intergranular cracking. The fracture dimple was still deep, and the mangle ridge was obvious, which indicated that the alloy had good performance at room temperature.
3.2 Microstructure
Fig.4 shows the TEM microstructures of inner-crystalline and inter-granular of Al-Cu-Mg-Ag alloys aged at 165 ℃ for 2 h and subsequently thermal exposed at 200 ℃ in different time. Uniform and fine Ω phases distributed dispersively in the matrix of alloys after ageing at 165 ℃ for 2 h and the microstructure of grain-boundary distributed continuously. It can be seen from Figs.4(b)-(f) that Ω phases coarsened at elevated temperature with the increasing of exposure time. The θ′ phase was found after thermal exposure for 20 h. After thermal exposure for 100 h, Ω phase grew up obviously. It was also found that there were granular second phases precipitated at grain boundary and the width of precipitation free zone(PFZ) on grain-boundary became larger.
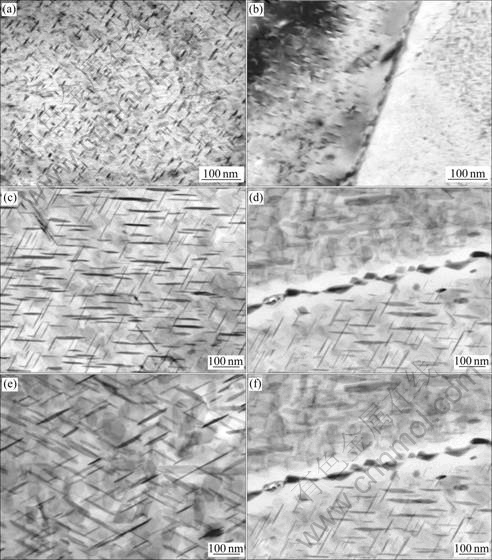
Fig.4 TEM microstructures at grain boundary of alloy after different thermal exposure conditions: (a) No thermal exposure; (b) No thermal exposure, at grain boundary; (c) 200 ℃, 20 h; (d) 200 ℃, 20 h, at grain boundary; (e) 200 ℃, 100 h; (f) 200 ℃, 100 h, at grain boundary
Fig.5 shows the TEM microstructures of Al-Cu-Mg-Ag alloy aged at 165 ℃ for 2 h and subsequently thermal exposed at 200 ℃ with a stress of 220 MPa in different time. It was observed that Ω phase begun to coarsen at elevated temperature with the increasing of exposure time (Figs.5(a) and (c)). Theθ′ phase precipitated after exposure for 20 h and Ω phase grew up obviously after 100 h.
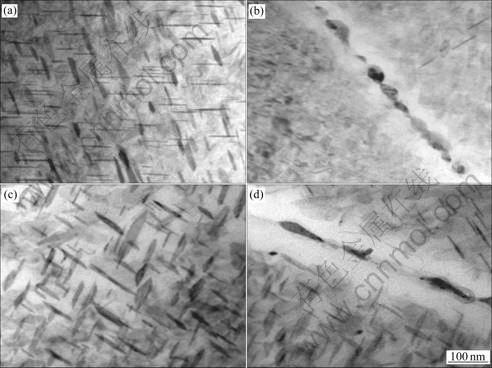
Fig.5 TEM microstructures of under-aged alloy after exposure at 200 ℃ and 220 MPa: (a) 200 ℃, 20 h; (b) 200 ℃, 20 h, at grain boundary; (c) 200 ℃, 100 h; (d) 200 ℃, 100 h, at grain boundary
The nucleation, growth and coarsening of precipitated phases in aluminum alloy were a essential process of diffusion of the solute atom and vacancy. The temperature was the main influencing factor during this process. The higher the ageing temperature, the higher the diffusion velocity of the solute atom and hence coarse equilibrium phase easily precipitated. On the contrary, the lower the ageing temperature, the lower the diffusion velocity of the atom, thus increasing the driving force of phase transformation, and the precipitated phases behaved high density and small size, which was good for alloy strengthening.
The tiny Ω phase in under-aged alloy continued to grow up under the effect of temperature and the stress.
This phenomenon may result from higher temperature, causing the diffusion easier to carry out, and the Ω phase could grow directly. It followed that the aging kinetics would be accelerated at high temperature.
It can be seen from Figs.5(b)-(d) that the granular second phases precipitated at grain boundary, and the width of precipitation free zone (PFZ) along grain boundary increased with exposure time.
Under-aged state of the alloy could change into peak-aged state under the effects of temperature and stress, thus making the strength of alloy increase. The Ω phases grew up with prolonging the time. Meanwhile, the equilibrium θ′ phase precipitated and PFZ emerged at grain boundary. The mechanism of PFZ formation was usually interpreted by solute-depletion and vacancy- depletion. According to the theory of vacancy, when the ageing temperature was higher than the critical temperature of uniform nucleation, the concentration of solutes must be higher than a critical value, thus assuring the uniform nucleation of precipitated phase. There were a large number of free solutes in the inner-crystal of under-aged alloy; hence, there were not PFZ along the grain boundary. The Ω phases began to coarsen with the increasing of time. The growth of Ω phases will consume a great deal of solute. When solutes in the matrix near grain boundary were depleted, the new solutes only came from re-dissolution of metastable Ω phases near the grain boundary. With the growing of Ω phases, the dissolution of metastable Ω phases near grain boundary took place gradually, thus increasing the width of PFZ with the increasing of time. This phenomenon was also observed in other aluminum alloys. Therefore, the increase of PFZ width in the process of anaphase ageing was interpreted by the mechanism of solute-depletion.
The applied stress could accelerate the formation of solute clustering or GP zone, but retard the precipitation and growth of θ′ and Ω phases. There was a critical stress value which adjusted the evolution of microstructure in the process of ageing for Al-Cu-Mg-Ag alloy. It would be helpful for the formation of solute clustering or GP zone when the applied stress was higher than critical stress. In the course of Al-Cu alloy and Al-Cu-Mg-Ag alloy, introduction of the applied stress would lead to tropism of deposition phase. The action of tensile stress would cause an increase of stress energy in the system and increasing levels were different in different variants, which engendered the stress exposure effect[15]. A tiny phase of under-aged alloy would continue to grow up with tensile stress loaded. It would be helpful for θ′ phrase to separate under the effects of stress and temperature.
4 Conclusions
1) There is peak strength after thermal exposure at 200 ℃ for 10 h of the under-aged Al-Cu-Mg-Ag alloy. Its surplus strengths σb and σ0.2 are 439.12 MPa and 414.04 MPa, respectively, and corresponding elongation is 9.72%. After thermal exposure for 100 h, its surplus strengths σb and σ0.2 are 386.95 MPa and 349.92 MPa, respectively, and corresponding elongation is 8.32%. Compared with under-aged alloy, the mechanical properties of the alloy decrease slightly.
2) There is peak strength after thermal exposure at 200 ℃ for 20 h with a stress of 220 MPa. Its surplus strengths σb and σ0.2 are 454 MPa and 442 MPa, respectively. After thermal exposure for 100 h, its surplus strengths σb and σ0.2 are 401 MPa and 391 MPa, respectively. The corresponding elongation is 9.7%. The mechanical properties of the alloy do not fall obviously, which indicates that the alloy has the excellent thermal stability.
3) The tiny Ω phase dispersion distributed in the matrix of under-aged alloys. There are θ′ and Ω phases precipitated competitively with the extending of time under a stress of 220 MPa. The width of PFZ increases and the granular second phases precipitate at grain boundary.
References
[1] POLMEAR I J, COUPER M J. Design development of an experimental wrought aluminum alloy for use at elevated temperatures [J]. Metall Trans A, 1988, 19A(4): 1027-1034.
[2] MUDDLE B C, POLMEAR I J. Precipitate Ω phase in Al-Cu-Mg-Ag alloys [J]. Acta Metallurgica, 1989, 37(3): 777-789.
[3] SCOTT V D, KERRY S, TRUMPER R L. Nucleation and growth of precipitates in Al-Cu-Mg-Ag alloys [J]. Materials Science and Technology, 1987, 3(10): 827-835.
[4] RINGER S P, HONO K, POLMEAR I J, SAKURAI T. Nucleation of precipitates in aged Al-Cu-Mg-(Ag) alloys with high Cu:Mg ratios[J]. Acta Materialia, 1996, 44(5): 1883-1898.
[5] POLMEAR I J, PONS G, BARBAUX Y, OCTOR H, SANCHEZ C, MORTON A J, BORBIDGE W E, ROGERS S. After Concorde: evaluation of creep resistant Al-Cu-Mg-Ag alloys [J]. Materials Science and Technology, 1999, 15: 861-868.
[6] TELESHOV V V, KAPUTKIN E Y, GOLOVLEVA A P, KOSMACHEVA N P. Temperature rangers of phase transformation and mechanical properties of alloys of the Al-Cu-Mg-Ag system with various Cu/Mg ratios [J]. Metal Science and Heat Treatment, 2005, 47(3/4): 139-144.
[7] ZHU Ai-wu, STARKE E A J, SHIFLET G J. An FP-CVM calculation of pre-precipitation clustering in Al-Cu-Mg-Ag alloys[J]. Scripta Materialia, 2005, 53: 35-40.
[8] LI Yun-tao, LIU Zhi-yi, LIU Yan-bin, XIA Qing-kun. Alloying behavior of rare-earth Er in Al-Cu-Mg-Ag alloy [J]. Materials Science Forum, 2007, 546/549(2): 941-946.
[9] LI Yun-tao, LIU Zhi-yi, XIA Qing-kun, LIU Yan-bin. Effect of trace addition of Er on microstructure and ageing behavior of Al-Cu-Mg-Ag-Zr alloy [J]. Transactions of Materials and Heat Treatment, 2007, 28(2): 49-53.
[10] LUMLEY R N, MORTON A J, POLMEAR I J. Enhanced creep performance in an Al-Cu-Mg-Ag alloy through underageing [J]. Acta Materialia, 2002, 50: 3597-3608.
[11] LUMLEY R N, POLMEAR I J. The effect of long term creep exposure on the microstructure and properties of an underaged Al-Cu-Mg-Ag alloy [J]. Scripta Materialia, 2004, 50: 1227-1231.
[12] KAZANJIAN S M, WANG N, STARKE E A J. Creep behavior and microstructural stability of Al-Cu-Mg-Ag and Al-Cu-Li-Mg-Ag alloy [J]. Materials Science and Engineering A, 1997, A234/236: 571-574.
[13] WANG J, WU X, XIA K. Creep behavior at elevated temperatures of an Al-Cu-Mg-Ag alloy [J]. Materials Science and Engineering A, 1997, A234/236: 287-290.
[14] SKROTZKI B, SHIFLET G J, STARKE A J. On the effect of stress on nucleation and growth of precipitates in an Al-Cu-Mg-Ag alloy [J]. Metallurgical and Materials Transactions A, 1996, 27A: 3431-3444.
[15] CHEN Da-qin, ZHENG Zi-qiao, LI Shi-chen, CHEN Zhi-guo. Mechanism of stress aging in Al-Cu(-Mg-Ag) alloys [J]. Trans Nonferrous Met Soc China, 2004, 14(4): 779-784.
[16] MURAISHI S, KUMAI S, SATO A. Competitive nucleation and growth of {111} Ω with {001} GP zone and θ′ in a stress-aged Al-Cu-Mg-Ag alloy [J]. Materials Transactions, 2004, 45(10): 2974-2980.
[17] LU Zheng, QIANG Jun, WU Yi-lei, LI Yong-wei, LIU Bo-cao. Effects of thermal exposure on properties of Al-Li 8090 extrudates [J]. Ordnance Material Science and Engineering, 1996, 19(4): 25-29.
Foundation item: Project(2005CB623705-04) supported by the National Basic Research Program of China
Corresponding author: LIU Zhi-yi; E-mail: liuzhiyi@mail.csu.edu.cn
(Edited by Ll Xiang-qun)