


Trans. Nonferrous Met. Soc. China 22(2012) 380-385
Low-temperature NO2 sensors based on polythiophene/WO3 organic-inorganic hybrids
GUO Xian-zhi, KANG Yan-fei, YANG Tai-li, WANG Shu-rong
TKL of Metal- and Molecule-based Material Chemistry, Key Laboratory of
Advanced Energy Materials Chemistry (MOE), Department of Chemistry, Nankai University, Tianjin 300071, China
Received 13 April 2011; accepted 23 June 2011
Abstract: Polythiophene (PTP) was prepared by a chemical oxidative polymerization and nanosized WO3 was prepared by a colloidal chemical method. The organic–inorganic PTP/WO3 hybrids with different mass fractions of PTP were obtained by a simple mechanically mixing the prepared PTP and WO3. The as-prepared PTP/WO3 hybrids have a higher thermal stability than the pure PTP. The gas sensing measurements demonstrate that the PTP/WO3 hybrid sensors exhibit higher response for detecting NO2 at low temperature than the pure PTP and WO3 sensor. The sensing mechanism is suggested to be related to the existence of p–n heterojunctions in the PTP/WO3 hybrids. The response of the PTP/WO3 hybrids is markedly influenced by the PTP mass fraction. The 20% PTP/WO3 hybrid shows high response and good selectivity to NO2 at low temperature (<90 oC). Therefore, the PTP/WO3 hybrids can be expected to be potentially used as gas sensor material for detecting NO2 at low temperature.
Key words: NO2 sensor; polythiophene/WO3; low temperature
1 Introduction
As one of the potential conducting polymers, PTP and its derivatives have attracted considerable attention for their easy polymerization and good environmental and thermal stability [1, 2]. However, there are also some disadvantages such as low chemical stability and mechanical strength that are unfavorable for conducting polymer-related applications.
WO3, an n-type semiconductor metal oxide, has received considerable attention for use as chemical sensor because of its unique sensing properties for a series of target gases, including H2S [3, 4], NO2 [5, 6], H2 [7], O3 [8], NH3 [9] and volatile organic compounds (VOCs) [10]. NO2 is toxic itself, badly harmful to human life and health, and, furthermore, is a main source of acid rain and photochemical smog [11]. WO3 has been considered a promising sensing material of solid-state semiconductor gas sensors for NO2 monitoring because of its excellent sensitivity [12]. However, similar to other semiconductor metal oxides, the high operating temperature and bad selectivity of WO3 for detecting NO2 restrict its actual application.
Inorganic–organic metal oxide/conducting polymer hybrid materials are currently of great interest for exploring enhanced sensor characteristics, due to their synergetic or complementary behaviors that are not available from their single counterparts [13]. Some effort has been paid to investigating this kind of hybrids for gas sensor applications. GUERNION et al [14] reported that the 3-alkylpolypyrrole-tin oxide composites exhibited much higher sensitivity and better selectivity to volatile organic compounds than the pure inorganic and organic materials. DESHPANDE et al [15] have synthesized the tin oxide-intercalated polyaniline nanocomposite, which had better sensitivity than the SnO2 and polyaniline with respect to ammonia gas exposure. HOSONO et al [16] prepared intercalated polypyrrole/MoO3 hybrids, which showed good sensing properties to VOCs.
In the present work, we prepared an organic- inorganic hybrid material containing PTP as the organic part and WO3 nanocrystalline powders as the inorganic part by a simple mechanically mixing the prepared PTP and WO3. The gas sensors based on the hybrids were fabricated and examined for gas sensing application for detecting NO2. Obtained results showed that the hybrid materials exhibited high response and good selectivity for detecting NO2 at low temperature.
2 Experimental
2.1 Preparation of PTP
PTP polymer was prepared via an in situ chemical oxidative polymerization. A specific amount of anhydrous FeCl3 (A.R., Tianjin Guangfu Fine Chemical Research Institute) was added into 50 mL ethyl glycol methyl ether (A.R., Tianjin Guangfu Fine Chemical Research Institute) under vigorous stirring. Then a certain volume of thiophene (TP) monomers (A.R., Tianjin Guangfu Fine Chemical Research Institute), with a mole ratio of TP to FeCl3 (1:3), was injected into the above stirred solution. After the reaction was carried out at room temperature for 3 h, the mixture was filtered. The obtained black precipitate was dipped in methanol for 24 h. The product was filtered and washed with methanol several times. The final product was dried at room temperature under vacuum for 24 h.
2.2 Preparation of WO3
WO3 was synthesized by a colloidal chemical method. Na2WO4·2H2O (A.R., Tianjin Guangfu Fine Chemical Research Institute) was dissolved into a certain amount of deionized water. Then a certain concentration of aqueous solution of HCl was drop-wise added to the sodium tungstate solution under stirring at room temperature till no white precipitate was further formed. The pH of the solution was adjusted with an aqueous solution of HCl in the reaction process. Then the solution was aged for 24 h, after which 15 mL of 0.15 mol/L cetyltrimethyl ammoniumbromide (CTAB) (A.R., Tianjin Guangfu Fine Chemical Research Institute) solution was immediately added. The abundant white flocculent precipitate formed was treated by ultrasonication for 40 min. Then the precipitate was filtrated and centrifuged with deionized water to remove Cl-, Br- and any other possible remnants, dried at 80 ?C and calcined at 600 ?C for 2 h. Light yellow WO3 powders were obtained.
2.3 Preparation of PTP/WO3 hybrids
A series of PTP/WO3 hybrids were prepared by mechanically mixing the above obtained PTP and WO3 with the PTP mass fraction of 5%, 10%, 20% and 30%, respectively, denoted as PW-5, PW-10, PW-20 and PW-30.
2.4 Characterization
X-ray diffraction (XRD) analyses were performed on Rigaku D/max-2500 diffractometer operating at 40 kV and 100 mA, using Cu Kα radiation (scanning range 2θ: 10°-80°). Fourier transfer infrared (FTIR) spectra were recorded using Avatar380FT-IR spectrometer series in the wave number range of 4000-400 cm-1. Thermal- gravimetry (TG) analyses were performed by ZRY-2P thermal analyzer at a linear heating rate of 10 °C/min. α-Al2O3 was used as reference.
2.5 Gas sensing properties test
The gas sensing measurements were performed on a commercial HW-30A system (a computer-controlled static gas sensing characterization system, Han Wei Electronics Co., Ltd., Henan Province, China). A proper account of sample powder was lightly grinded with several drops of terpineol in an agate mortar to form slurry. Then, the slurry was coated onto the outside surface of the alumina tube with 4 mm in length and 1 mm in diameter, as well as containing two Au electrodes and four Pt wires on both ends of the tube. A small Ni-Cr alloy filament was put through the tube to supply the operating temperatures by tuning the heating voltage. Finally, the Al2O3 tube was welded onto a pedestal with six probes. The sensor response to NO2 gas is defined as the ratio of Rg/Ra, where Rg and Ra are the electrical resistances of the sensor in NO2 gas and in air, respectively.
3 Results and discussion
Figure 1 shows XRD pattern of the prepared WO3. All the diffraction peaks can be well indexed to orthorhombic WO3 (JCPDS file no. 20—1324). No peaks for other impurities can be detected, indicating the formation of pure WO3. The sharp peaks suggest that the crystal of WO3 is perfect. The average crystallite size of WO3 particles is about 29 nm, calculated by Scherrer’s equation.
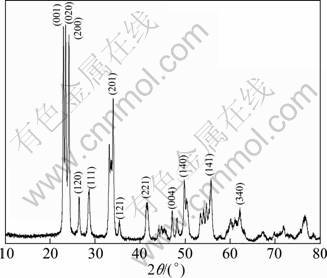
Fig. 1 XRD pattern of prepared WO3
FTIR spectrum of the prepared PTP is shown in Fig. 2. Several weak peaks in the range of 2800-3100 cm-1 and 1630 cm-1 can be attributed to the C—H stretching vibrations and C=C characteristic peak, respectively. In the range of 600-1500 cm-1, it is the fingerprint region of PTP. The peak at 784 cm-1 is due to the C—H out-of-plane vibration of the 2, 5-substituted thiophene ring created by the polymerization of thiophene monomer. The peak at approximately 695 cm-1 denotes the C—S stretching in the thiophene ring [17-20].
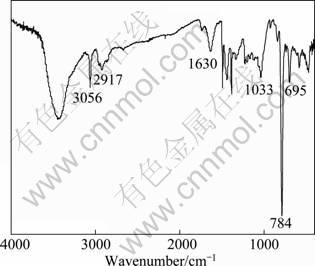
Fig. 2 FT-IR spectrum of prepared PPT
Thermal stability of the hybrid was examined by TG analysis. Figure 3 presents the TG curves of the prepared pure PTP and PW-20 hybrid. In the case of the pure PTP (Fig. 3(a)), the mass starts to decrease at approximately 170 °C, and almost decomposes completely when the temperature is up to 750 °C. Different from the pure PTP, the PW-20 hybrid is still stable at the temperature below 300 °C. The delay of decomposition process of the hybrid indicates that the thermal stability of the PTP/WO3 hybrids is better than that of the pure PTP. As a result, these data confirm that the mass fraction of WO3 in the PTP/WO3 hybrids is responsible for the higher thermal stability of the hybrids, which is beneficial for the potential application of the hybrids as chemical sensors.
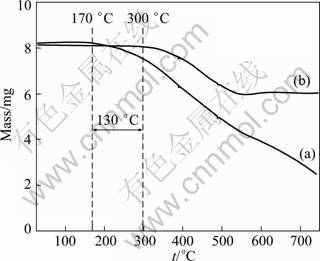
Fig. 3 TG curves of prepared pure PTP (a) and PW-20 hybrid (b)
To investigate the gas-sensing properties of the prepared PTP/WO3 hybrids to NO2 at low temperature, the gas-sensing test was carried at room temperature (RT), 60 and 90 °C, respectively. The gas sensing results are included in Figs. 4-8.
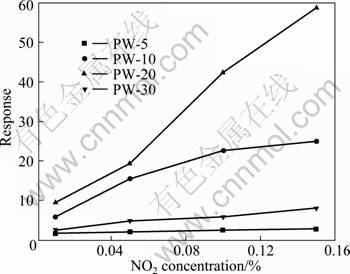
Fig. 4 Response of prepared PTP/WO3 hybrids with different PTP mass fractions to NO2 with different concentrations at room temperature
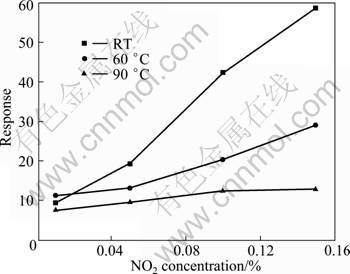
Fig. 5 Response of PW-20 hybrid to NO2 with different concentrations at different operating temperatures
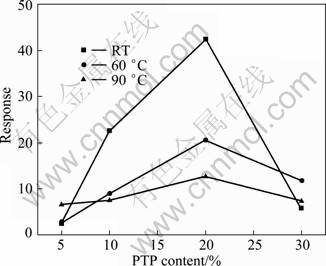
Fig. 6 Response of PTP/WO3 hybrids with different PTP contents to 0.1% NO2 (volume fraction) at different operating temperatures
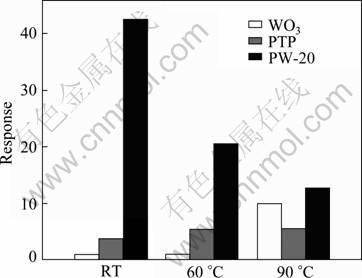
Fig. 7 Response of PW-20 hybrid, pure WO3 and pure PTP to 0.1% NO2 (volume fraction) at different operating temperatures
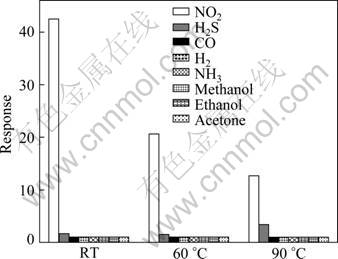
Fig. 8 Response of PW-20 hybrid to various gases with same concentration of 0.1% at different operating temperatures
Figure 4 shows the response of the PTP/WO3 hybrids with different PTP mass fractions (5%, 10%, 20% and 30%) to different concentrations (0.01%, 0.05%, 0.1% and 0.15%, volume fraction) of NO2 at room temperature. All the four PTP/WO3 hybrids show a similar response pattern to NO2. From Fig. 4, it can be concluded that the gas response is a function of NO2 concentration. The gas response increases generally to a certain extent with an increase in concentration of NO2 from 0.01% to 0.15% (volume fraction). The maximum response can be observed when the NO2 gas concentration is 0.15% (volume fraction). Meanwhile, the response of PTP/WO3 hybrids enhances with the increase of PTP mass fraction from 5% to 20%, and the 20% PTP/WO3 hybrid shows the highest response to NO2. However, further increase of the PTP mass fraction results in the decrease of the response.
Figure 5 illustrates the response of the PW-20 hybrid to NO2 with different concentrations at different operating temperatures. It can be observed that, similar to at room temperature, at the operating temperature of 60 and 90 °C the response increases with the increase of NO2 concentration, and all the hybrids show the maximum response to 0.15% NO2 (volume fraction). It is also found that the PTP/WO3 hybrid exhibits much higher response to NO2 at RT than at 60 or 90 °C. Therefore, it is suggested that this kind of hybrids can be potentially used for gas sensor material for detecting NO2 at RT.
Figure 6 shows the response of 5%, 10%, 20% and 30% PTP/WO3 hybrids to 0.1%NO2 (volume fraction) at RT, 60 or 90 °C. It is found that the PW-20 hybrid has higher response to 0.1%NO2 (volume fraction) at RT than at 60 °C or 90 °C, and at all the three operating temperatures, the PW-20 hybrid shows the highest response among the PTP/WO3 hybrids.
Figure 7 presents the response of the PW-20 hybrid, pure WO3 and pure PTP to 0.1% NO2 (volume fraction) at different operating temperatures. Obviously, compared with the pure WO3 and PTP, the sensor response has been significantly improved by constructing hybrid materials. Therefore, the PTP/WO3 hybrid can be one of the most promising materials due to its high response at low temperature. It is well known that PTP behaves as a p-type semiconductor and WO3 behaves an n-type semiconductor. It is expected that the p–n heterojunctions are formed in the PTP/WO3 hybrids [21], which could generate a unique electron donor–acceptor system, increasing the depletion barrier height and thus improving the response of the sensor [22]. The test gas can adjust the conductivity of the junction by changing the depletion region. When the NO2 gas is introduced, the width of the depletion region decreases and the conductivity of the PTP channel increases [23-25]. Therefore, the PTP/WO3 hybrid shows much higher response in comparison to pure PTP and WO3 at low operating temperature. However, it can also be observed from Figs. 4-6 that the concentration of the PTP is as high as 30% which leads to the decrease in the response of the PTP/WO3 to NO2, which should be related to the fact that the p-n heterojunctions are covered with the excessive PTP. As a result, the effective p-n heterojunctions are reduced and the response decreases.
For practical use, the selectivity of the chemical sensor is also an important consideration. Figure 8 shows the response of the PW-20 hybrid to various gases including NO2, H2S, CO, H2, NH3, ethanol, methanol and acetone with the same concentration of 0.1% NO2 (volume fraction) at RT, 60 and 90 °C, respectively. It is found in Fig. 8 that the PW-20 hybrid exhibits high response to NO2, but less to H2S, and no gas response to CO, H2, NH3, ethanol, methanol and acetone, illuminating that the sensor based on the as-obtained PTP/WO3 hybrid has good selectivity to NO2.
4 Conclusions
1) The as-prepared PTP/WO3 hybrids have a higher thermal stability than the pure PTP, confirming that the presence of WO3 in the PTP/WO3 hybrid is responsible for the higher thermal stability of the hybrid, due to a certain synergetic interaction between the inorganic WO3 and organic PTP components, which is beneficial for the potential application of the hybrid as chemical sensors.
2) The gas sensing measurements demonstrate that the sensors based on the PTP/WO3 hybrids exhibit higher gas response for detecting NO2 at low temperature than the sensor based on pure PTP or WO3. The sensing mechanism is suggested to be related to the existence of p–n heterojunctions in the PTP/WO3 hybrids.
3) The response of the PTP/WO3 hybrids is markedly influenced by the PTP mass fraction. Among the hybrids, the 20% PTP/WO3 hybrid shows the highest response to NO2. Moreover, the 20% PTP/WO3 hybrid shows higher response at room temperature than at 60 or 90oC. Meanwhile, the PTP/WO3 hybrid exhibits much higher sensor response to NO2 compared with other gases including H2S, CO, H2, NH3, ethanol, methanol and acetone, illuminating that the sensors based on the as-obtained PTP/WO3 hybrids have good selectivity to NO2.
References
[1] ANDERSON N N, HAO E, AI X, HASTINGS G, LIAN T. Ultrafast and long-lived photoinduced charge separation in MEH-PPV/nanoporous semiconductor thin film composites [J]. Chem Phys Lett, 2001, 347: 304-310.
[2] MARSELLA M J, CARROLL P J, SWAGER T M. Design of chemoresistive sensory materials: Polythiophene-based pseudopolyrotaxanes [J]. J Am Chem Soc, 1995, 117: 9832-9841.
[3] PENZA M, TAGLIENTE M A, MIRENGHI L, GERARDI C, MARTUCCI C, CASSANO G. Tungsten trioxide (WO3) sputtered thin films for a NOx gas sensor [J]. Sens Actuators B, 1998, 50: 9-18.
[4] FRUHBERGER B, GRUNZE M, DWYER D J. Surface chemistry of H2S sensitive tungsten oxide films [J]. Sens Actuators B, 1996, 31: 167-174.
[5] WANG X, MIURA N, YAMAZOE N. Study of WO3-based sensing materials for NH3 and NO detection [J]. Sens Actuators B, 2000, 66: 74-76.
[6] CHOI Y, SAKAI G, SHIMANOE K, MIURA N, YAMAZOE N. Wet process prepared hick film of WO3 for NO2 [J]. Sens Actuators B, 2003, 95: 258-265.
[7] IPPOLITOA S J, KANDASAMY S, KALANTAR-ZADEHA K, WLODARSKIA W. Hydrogen sensing characteristics of WO3 thin film conductometric sensors activated by Pt and Au catalysts [J]. Sens Actuators B, 2005, 108: 154-158.
[8] UTEMBE S R, HANSFORD G M, SANDERSON M G, FRESHWATER R A, PRATT K F E, WILLIAMS D E, COX R A, JONES R L. An ozone monitoring instrument based on the tungsten trioxide (WO3) semiconductor [J]. Sens Actuators B, 2006, 114: 507-512.
[9] SRIVASTAVA V, JAIN K. Highly sensitive NH3 sensor using Pt catalyzed silica coating over WO3 thick films [J]. Sens Actuators B, 2008, 133: 46-52.
[10] KANDA K, MAEKAWA T. Development of a WO3 thick-film-based sensor for the detection of VOC [J]. Sens Actuators B, 2005, 108: 7-10.
[11] HAN X, NAEHER L P. A review of traffic-related air pollution exposure assessment studies in the developing world [J]. Environ Int, 2006, 32: 106-120.
[12] JIMENEZ I, ARBIOL J, DEZANNEAU G, CORNET A, MORANTE J R. Crystalline structure, defects and gas sensor response to NO2 and H2S of tungsten trioxide nanopowders [J]. Sens Actuators B, 2003, 93: 475-485.
[13] SCHNITZLER D C, MERUVIA M S, HUMMELGEN I A, ZARBIN A J G. Preparation and characterization of novel hybrid materials formed from (Ti, Sn)O2 nanoparticles and polyaniline [J]. Chem Mater, 2003, 15: 4658-4665.
[14] GUERNION N, de LACY COSTELLO B P J, RATCLIFFE N M. The synthesis of 3-octadecyl and 3-docosylpyrrole, their polymerisation and incorporation into novel composite gas sensitive resistors [J]. Synth Met, 2002, 128: 139-147.
[15] DESHPANDE N G, GUDAGE Y G, SHARMA R, VYAS J C, KIM J B, LEE Y P. Studies on tin oxide-intercalated polyaniline nanocomposite for ammonia gas sensing applications [J]. Sens Actuators B, 2009, 138: 76-84.
[16] HOSONO K, MATSUBARA I, MURAYAMA N, WOOSCK S, IZU N. Synthesis of polypyrrole/MoO3 hybrid thin film and their volatile organic compound gas-sensing properties [J]. Chem Mater, 2005, 17: 349-354.
[17] ZHU Yun-feng, XU Shou-bin, JIANG Long, PAN Ke-liang, DAN Yi. Synthesis and characterization of polythiophene/titanium dioxide composites [J]. React Funct Polym, 2008, 68: 1492-1498.
[18] KARIM M R, LEE C J, LEE M S. Synthesis and characterization of conducting polythiophene/carbon nanotubes composites [J]. J Polym Sci Part A, 2006, 44: 5283-5290.
[19] KARIM M R, LIM K T, LEE C J, LEE M S. A facile synthesis of polythiophene nanowires [J]. Synth Met, 2007, 157: 1008-1012.
[20] HAN M G, FOULGER S H. Crystalline colloidal arrays composed of poly-(3,4-ethylenedioxythiophene)-coated polystyrene particles with a stop band in the visible regime [J]. Adv Mater, 2004, 16: 231-234.
[21] AN X, ANDERSON N, GUO J C, KOWALIK J, TOLBERT L M, LIAN T Q. Ultrafast photoinduced charge separation dynamics in polythiophene/SnO2 nanocomposite [J]. J Phys Chem B, 2006, 110: 25496-25503.
[22] XU Mi-juan, ZHANG Jun, WANG Shu-rong, GUO Xia-zhi, XIA Hui-juan, WANG Yan, ZHANG Sou-min, HUANG Wei-ping, WU Shi-hua. Gas sensing properties of SnO2 hollow spheres/ polythiophene inorganic–organic hybrids [J]. Sens Actuators B, 2010, 146: 8-13.
[23] RAM M K, YAVUZ O, ALDISSI M. NO2 gas sensing based on ordered ultrathin films of conducting polymer and its nanocomposite [J]. Synth Met, 2005, 151: 77-84.
[24] JAIN K, PANT R P, LAKSHMIKUMAR S T. Effect of Ni doping on thick film SnO2 gas sensor [J]. Sens Actuators B, 2006, 113: 823-829.
[25] POTJE-KAMLOTH K. Semiconductor junction gas sensors [J]. Chem Rev, 2008, 108: 367-399.
基于聚噻吩/WO3的有机-无机复合材料低温NO2传感器
郭先芝,康艳飞,杨太利,王淑荣
南开大学 化学系,先进能源材料化学教育部重点实验室,金属与分子基材料化学天津市重点实验室,天津 300071
摘 要:通过化学氧化聚合法和胶溶法分别制备了聚噻吩(PTP)和纳米WO3,通过简单机械共混和PTP和WO3制备不同PTP质量分数的有机-无机PTP/WO3 复合物。所制备的PTP/WO3复合物比纯的PTP具有更高的热稳定性。气敏测试结果表明,在低温检测NO2时,PTP/WO3复合物具有比纯的PTP和WO3 更高的响应,这可能与PTP/WO3复合物中p–n异质节的存在有关系。PTP/WO3复合物的响应受PTP质量分数的影响显著,20% PTP/WO3复合物在低温(<90 °C)时对NO2 显示出高的响应和好的选择性。因此,PTP/WO3传感器有望被用于低温检测NO2
关键词:NO2传感器;聚噻吩/WO3;低温
(Edited by LI Xiang-qun)
Foundation item: Project (21171099) supported by the National Natural Science Foundation of China; Projects (09JCYBJC03600, 10JCYBJC03900) supported by Technology Commission Foundation of Tianjin, China
Corresponding author: WANG Shu-rong; Tel: +86-22-23505896; Fax: +86-22-23502458; E-mail: shrwang@nankai.edu.cn
DOI: 10.1016/S1003-6326(11)61187-4