J. Cent. South Univ. Technol. (2009) 16: 0365-0370
DOI: 10.1007/s11771-009-0062-y
Improvement of surface hydrophobicity on silicone rubber modified by CF4 radio frequency capacitively coupled plasma
GAO Song-hua(高松华)1, ZHOU Ke-sheng(周克省)1, WEN Li-shi(闻立时)1, 2, 3
(1. School of Physics Science and Technology, Central South University, Changsha 410083, China;
2. School of Materials Science and Engineering, Dalian University of Technology, Dalian 116024, China;
3. Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China)
Abstract: In order to improve the surface hydrophobicity, silicone rubber (SIR) samples were exposed to CF4 radio frequency (RF) capacitively coupled plasma (CCP). Attenuated total reflection Fourier transform infrared (ATR-FTIR) spectrum and X-ray photoelectron spectroscopy (XPS) were used to observe the variation of the functional groups of the modified SIR. Static contact angle (SCA) was employed to estimate the change of hydrophobicity of the modified SIR. The surface energy of SIR is reduced largely from 27.37 mJ/m2 of original SIR sample to 2.94 mJ/m2 of SIR sample treated by CF4 CCP modification at RF power of 200 W for a treatment time of 5 min. According to the XPS, ATR-FTIR and surface energy analysis, it is suggested that the improvement of hydrophobicity on the modified SIR surface is mainly ascribed to the decrease of surface energy, which is caused by the cooperation of the fluosilicic structure of Si—F or Si—F2 and the fluoric groups of C—CFn induced by the methyl replacement reaction and residual methyl groups of SIR surface.
Key words: surface hydrophobicity; silicone rubber; radio frequency capacitively coupled plasma; static contact angle
1 Introduction
Electrical insulators are very important components in the electrical power system, such as substation and distribution, and transmission lines. However, pollution flashovers have become the major impediment to the uninterrupted supply of electrical power. On traditional glass and porcelain insulator surfaces, wet atmospheric conditions easily lead to the appearance of water films, and then salts in the contaminants dissolve into the water films, which would result in uncontrolled leakage currents and flashovers. So the maintenance of outdoor insulators has been an art learned through experience with many methods. One of the best methods to improve insulating properties of the insulator, particularly in wet and heavily polluted conditions, is to utilize the silicone rubber (SIR). Owing to its excellent electric characters, such as electrical erosion resistance, migration of hydrophobicity and pollution flashover resistance [1], SIR is used increasingly as outdoor high voltage insulators because one property of the SIR insulators is the ability to restore the surface hydrophobicity after a pollution layer has built up on the surface, which can suppress the development of leakage currents, dry band arcing and flashover. However, SIR insulators suffer from loss of hydrophobicity, decreased tracking and erosion resistance, and degradation of their surface under heavy wet atmospheric conditions such as heavy fog, drizzling rain and acid rain. If the SIR’s surface has the properties of anti-adhesion, low surface energy and super-hydrophobicity, it can reduce the solid contamination adhesion, so that surface leakage currents and pollution flashovers due to the contaminations deposited on the surface would be suppressed efficiently.
Up to now, some reports have described that modified polydimethylsiloxane (PDMS) surfaces were performed for some specific purposes. For example, KHORASANI and MIRZADEH [2] made PDMS surfaces superhydrophobic and superhydrophilic to serve as biomedical materials in vitro blood compatibility. GARRA et al [3] reported the etching effects of O2 and CF4 mixture plasma on PDMS. RANGEL et al [4] applied sulphur hexafluoride (SF6) plasmas and argon plasma immersion ion implantation (ArPIII) techniques to improve the hydrophobicity of poly(tetrafluoroethylene) (PTFE), polyurethane and silicone surfaces. However, there are few reports about the SIR surface modification because researchers in the field of insulators still pay much attention to the composition modification of bulk materials via adding fillers to SIR and the study of surface hydrophobicity transfer [5-6]. Although the fillers, which are always alumina tri-hydrate (Al2O3·3H2O) and silicon oxide (SiO2)added into SIR, can improve its tracking and erosion resistance, and hardness, resulting in the decrease of SIR hydrophobicity as well.
Therefore, for the sake of further increasing the resistance to pollution flashover and prolonging the service life of SIR as outdoor high voltage insulators, surface modification is required to improve the hydrophobicity of SIR surface while preserving bulk properties. Plasma treatment has proven to be a powerful tool toward surface modification of polymeric materials [7-9]. By using plasma treatment, interfacial properties can be introduced to a polymer surface without affecting the desired bulk properties of a material, such as strength, and toughness [10-11]. There are many methods to make fluorinated polymer surface, such as fluorine-containing electrical discharges [12], plasma polymerization of fluoromonomers [13], sputter deposition from a polytetrafluorethylene (PTFE) target [14], fluorinated thin films deposited by radio frequency plasma enhanced chemical vapor deposition (RF-PECVD) [15], and chemical derivatization [16]. Especially, the employment of CF4 plasma treatment to introduce fluorine groups onto polymer surface is an effectual way to lower surface adhesiveness, surface energy and coefficient of friction without creating a new interface between the bulk and the new layer, which would lead to the out-of-control in the polymer’s electric properties [11-12, 17-20].
In this work, to obtain the high hydrophobicity effect without weakening bulk properties, SIR samples used as outdoor insulators were treated by CF4 radio frequency (RF) capacitively coupled plasma (CCP) at a RF power of 60-200 W and a CF4 flow rate of 3.33×10-7 m3/s for treatment time up to 20 min, respectively. The variable process of hydrophobicity and the change of surface chemical composition were analyzed through water static contact angle (SCA) testing technique, the attenuated total reflection Fourier transform infrared (ATR-FTIR) spectrum and X-ray photoelectron spectroscopy (XPS) technique. And the reasons for the improvement of hydrophobic property on modified SIR surface were discussed.
2 Experimental
2.1 Preparation of materials
The SIR samples (1.0 cm×1.0 cm×0.2 cm) were obtained from Dongguan Gaoneng Industrial Co. Ltd., China. Generally, SIR samples consist of a polymer and fillers that are alumina tri-hydrate (Al2O3·3H2O) and silicon oxide (SiO2) added to SIR to improve its tracking and erosion resistance, and hardness. The PDMS is the basic polymer of SIR samples. A typical PDMS structure is shown in Fig.1. The samples were soaked with acetone and ethanol for 10 h, respectively, then washed repeatedly with deionized water, dried in an oven at 60 ℃ for 3 h, and finally stored in a drying desiccator before use.

Fig.1 Chemical structure of PDMS (n=100-1000)
2.2 Plasma treatment
The plasma treatments of the SIR samples were carried out in a RF CCP reactor (Fig.2.) operating at 13.56 MHz. The CCP reactor consists of two cylindrical electrodes of 20 cm in diameter and 11 cm apart in a cylindrical vacuum vessel. The upper electrode and the vessel wall were grounded. The SIR samples were placed on the sample holder, where the SIR surfaces should be bombarded by positively charged ions accelerated as they traverse the plasma sheath adjacent to the electrode. After the system was evacuated to 2×10-3 Pa, CF4 gas was introduced into the vessel at a flow rate of 3.33×10-7 m3/s, and the operating pressure was subsequently maintained at a treatment pressure of 10 Pa. The RF plasma was then generated at the specified electric power of 60, 100 and 200 W, respectively, for a desired treatment period, up to 20 min. After plasma treatment, the modified samples were taken out and stored in a drying desiccator.
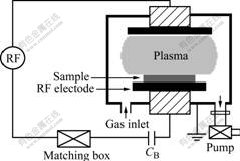
Fig.2 Schematic plan of CCP reactor (CB is direct current isolation capacitance)
2.3 Methods of characterization
SCA for distilled water was analyzed on a DSA100 drop shape analysis system (Kruss, Germany) via the sessile drop method. The volume of testing liquid drop was 4 μL and the dripping rate of testing liquid drop was 32.19 μL/min. The mean values reported correspond to three measurement points located uniformly on the surface. Chemical bonds at a depth of 1-10 μm can be identified by using ATR-FTIR. The ATR technique can be used to investigate the materials that block the penetration of light. The radiation in the ATR technique penetrated a few microns into the sample and then the radiation was reflected into the optical element. The equipment used was a FTIR-spectrometer, from Nicolet (America), model Nexus. XPS was employed to investigate the influence of hydrophobic property according to the variation of F content and composition of fluoric groups of the SIR samples’ surface. An ESCALAB250 system from Institute of Metal Research, Chinese Academy of Sciences, was used with a monochromatic Al Kα (hν=1 486.6 eV) X-ray source operating at a power of 300 W. X-ray beam with a circular cross-section area of 1 cm2 was irradiated onto sample at an incident angle of 45? with respect to the surface plane and the photoelectrons were detected at 90? takeoff angle. Photoelectrons were analyzed with a concentric hemispherical analyzer at a pass energy of 50 eV. A step-scan interval of 1 eV was used for wide scan, and 0.05 eV for high-resolution scans; acquisition time was 60 s at both resolutions. All binding energies were referenced to the C1s neutral carbon peak at 285.0 eV to compensate for surface-charging effects. Element stoichiometries were determined by the high-resolution peak areas using Shirley background subtraction.
3 Results and discussion
3.1 SCA analysis
Fig.3 shows evolution comparison of the SCA for distilled water at RF powers of 60, 100 and 200 W, respectively. It can be seen that there are different variation rules of water contact angle of modified SIR surface. At a RF power of 200 W, the water static contact angle of the modified SIR surface increases rapidly from 100.7? of the original sample to a maximum of 150.2? of 5 min-treated SIR sample, and further decreases gradually with the increase of treatment time. At a RF power of 100 W, the water contact angle also reaches a maximum, and then keeps basically the state in spite of the increase of treatment. However, at the lowest RF
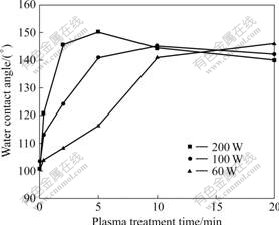
Fig.3 Evolution comparison of SCA for distilled water at different RF powers
power of 60 W, the water contact angle increases with the increase of treatment time up to 20 min. It is proposed that the competition between CF4 plasma fluorination and ablation or etching reaches the equilibrium more quickly at the higher RF power. With the increase of the RF power, the self-bias on the SIR samples being modified by CF4 CCP increases. From Fig.3, it can also be seen that the ablation or etching increases more quickly than fluorination so that the time to reach the equilibrium is shortened with the increase of the RF power.
3.2 ATR–FTIR analysis
Fig.4 shows that the absorption peaks of C—H (about 1 015 cm-1) and CH3 (about 1 260 cm-1) shrink rapidly, while the absorption peak of Si—O—Si (about 2 962 cm-1) shrinks lightly with the increase of treatment time. Fig.5 shows the variation in the ratio of the optical density between the above three peaks and Si—(CH3)2 peak (about 794 cm-1) with the increase of treatment time. The results indicate that the chemical bond of Si—CH3 is most easily broken by the positive ions from
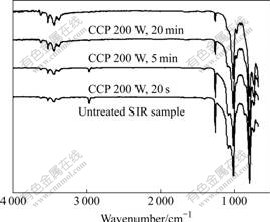
Fig.4 ATR-FTIR spectra evolution comparison at different treatment time

Fig.5 Ratio of optical density between other groups’ peaks and Si—(CH3)2 peak as function of plasma treatment time
the CF4 plasma due to the weak chemical bond dissociation energy between Si and CH3, and chemical bonds of Si—O and C—H are not easily broken owing to their higher chemical bond dissociation energies. It is suggested that the fluoric groups mainly replace the methyl groups and cooperate with the residual methyl groups linked to the Si atoms to improve the hydrophobicity of the modified SIR samples. In addition, the main reason for not finding fluoric groups in the ATR-FTIR spectra is that the signal of fluoric groups in the modification layer is much weaker than that of the substrate because the radiation in the ATR technique penetrates a few microns into the sample and then is reflected into the optical element. However, the depth of fluoric layer on modified SIR is only several atoms distances.
In order to investigate whether fluoric groups were introduced onto the SIR samples surface or not, XPS was used to verify the result because it is one of useful tools to detect chemical composition of the surface.
3.3 XPS analysis
PDMS has a theoretical mole ratio of C to Si to O of 2.0?1.0?1.0. XPS analysis gives the elemental mole ratio of C to Si to O of 1.8?1.1?1.0 for the original SIR sample, which is different from the theoretical ratio because of the fillers existing in the SIR.
The XPS survey spectra of original SIR sample and the SIR sample treated by CF4 CCP under RF power of 200 W for 5 min, are shown in Fig.6. According to the comparison between the original sample and 5 min treated sample, it is clear that F content increases obviously, while O content basically keeps the same state, and the contents of C and Si decrease by a small margin, respectively. Fig.7 shows that the F content increases monotonously and plateaus off with treatment time from 2 to 20 min. The increase of fluorine and the decrease of Si and C observed in the XPS spectra (Fig.6) are attributed to the rule, which the CF4 plasma fluorination and ablation or etching occurring on the polymer surface are parallel and competitive, and the competition between fluorination and ablation or etching is dependent on RF power and treatment time [21-22]. Under the constant RF power of 200 W, fluorination predominates over ablation or etching initially, however, beyond a critical treatment time, the two reactions reach a dynamic near-equilibrium with slowly varying F content on the surface.
Fig.8 shows the carbon (C1s) and fluorine (F1s) high-resolution XPS spectra of SIR sample treated by CF4 CCP for 5 min. There are two peaks in the C1s spectrum. One is present at 284.8 eV corresponding to the —Si(CH3)2—O—n species [23], and the other appears at 286.5 eV corresponding to C—CFn functional

Fig.6 XPS survey spectra of SIR samples treated by CF4 CCP under RF power of 200 W: (a) Original sample; (b) Treatment for 5 min
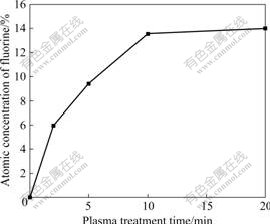
Fig.7 Surface fluorine content of SIR samples treated by CF4 CCP at RF power of 200 W for different treatment time
groups [10]. The reactive species in pure CF4 plasma are primarily fluorine atoms with a small concentration of complex fluorocarbon species [24]. Fluorine atoms and F-substituted methyl species (—CFxH(3-x), 0≤x≤3) can be grafted onto a polymeric surface via methyl or hydrogen replacement and opening of unsaturated bonds

Fig.8 XPS spectra of SIR sample treated by CF4 CCP at RF power of 200 W for 5 min: (a) C1s; (b) F1s
to form the Si—F and C—F functional groups. The methyl replacement by fluorine atom is highly probable, considering the lower chemical bond dissociation energy of C—Si in Si—CH3 than that of C—H in CH3, resulting in the F—Si component over F—C largely (Fig.8(b)).
The mole ratio of F—C to F—Si (n(F—C)/ n(F—Si)) as a function of CF4 plasma treatment time is shown in Fig.9. There is a dramatic decrease in the bond ratio from 0.248 1 to 0.089 7 with plasma treatment time from 2 to 5 min, and the bond ratio keeps the same state with the increase of treatment time from 5 to 20 min. The result is ascribed to the fact that the F atoms, which have been added to the Si atoms, are more difficult to be ablated by energetic positive ions than the functional groups of C—CFn added to the Si atoms, owing to much stronger chemical bond dissociation energy of Si—F than that of Si—C. Therefore, One or two F atoms can be added to one Si atom to replace one or two methyl groups linked to Si and form —SiFx(CH3)2-x—O—n (x=1, 2) structure. The Si—F or F—Si—F in the —SiFx(CH3)2-x—O—n (x=1, 2) structure is more stable than Si—CH3 in the —Si(CH3)2—O—n structure due to its higher chemical

Fig.9 n(F—C)/n(F—Si) as function of CF4 CCP treatment time at RF power of 200 W
bond dissociation energy, and the lower surface energy
can be acquired via Si—F or F—Si—F rotating and arranging in the —SiFx(CH3)2-x—O—n (x=1, 2) structure. According to the comparison among Figs.3, 7 and 9, the improvement of modified SIR surface hydrophobicity is not only relative to the increase of surface fluorine content, but also associated with the formation of the Si—F or F—Si—F structure. Hence, it is proposed that the great improvement of static contact angle of modified SIR surface is mainly ascribed to the cooperation of the fluosilicic structure of Si—F or F—Si—F and the fluoric groups of C—CFn induced by the methyl replacement reaction, and residual methyl groups of SIR surface.
3.4 Surface energy analysis
Surface energy of untreated SIR sample was calculated using measured contact angles to be 27.37 mJ/m2. It is higher than the reported value of 21.6 mJ/m2 for pure SIR due to the inclusion of fillers. As expected, CF4 plasma treatment decreases the surface energy of SIR modified at a RF power of 200 W (Fig.10). Its variation is opposite to the change of water static contact angle (Fig.3). The surface energy decreases rapidly within a short treatment time of 5 min, and the curve of surface energy vs CF4 plasma treatment time keeps relatively stable if the treatment time is longer than 5 min. Compared with the surface fluorine content variation of SIR samples treated by CF4 CCP (Fig.7), it is concluded that the increase of fluorine on the surface of SIR samples, which is mainly introduced through the reaction of F atoms replacing methyl groups to form the —SiFx(CH3)2-x—O—n (x=1, 2) structure, makes the surface energy decrease and the static contact angle increase.
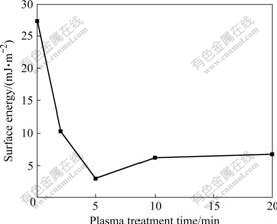
Fig.10 Surface energy of SIR samples modified at RF power of 200 W as function of CF4 plasma treatment time
4 Conclusions
(1) The fluorination and ablation or etching occur on the SIR surfaces at the same time when the SIR samples are treated by CF4 RF CCP, and the competition between fluorination and ablation or etching reaches the equilibrium more quickly at the higher RF power because the self-bias on the SIR samples increases with the increase of the RF power.
(2) According to XPS analysis results, which the mole ratio of F—C to F—Si on the modified SIR surface is very small and changes in the range of 0.067 3 to 0.248 1, the F atoms introduced onto the surface of SIR samples mainly exist in the —SiFx(CH3)2-x—O—n (x=1, 2) structure formed by the F replacement —CH3.
(3) The improvement of hydrophobicity on the modified SIR surface is mainly ascribed to the cooperation of the fluosilicic structure of Si—F or Si—F2, the fluoric groups of C—CFn, and residual methyl groups.
(4) The rule of surface energy variation of modified SIR supports the opinion that the lower surface energy increases surface chemical inertness and produces higher hydrophobic surface.
References
[1] CHERNEY E A, GORUR R S. RTV silicone rubber coatings for outdoor insulators [J]. IEEE Transactions on Dielectrics and Electrical Insulation, 1999, 6(5): 605-611.
[2] KHORASANI M T, MIRZADEH H. In vitro blood compatibility of modified PDMS surfaces as superhydrophobic and superhydrophilic materials [J]. Journal of Applied Polymer Science, 2004, 91(3): 2042-2047.
[3] GARRA J, LONG T, CURRIE J, SCHNEIDER T, WHITE R, PARANJAPE M. Dry etching of polydimethylsiloxane for microfluidic systems [J]. Journal of Vacuum Science & Technology A—Vacuum Surface and Films, 2002, 20(3): 975-982.
[4] RANGEL E C, BENTO W C A, KAYAMA M E, KAYAMA M E, SCHREINER W H, CRUZ N C. Enhancement of polymer hydrophobicity by SF6 plasma treatment and argon plasma immersion ion implantation [J]. Surface and Interface Analysis, 2003, 35(2):179-183.
[5] YOUN B H, HUH C S. Surface degradation of HTV silicone rubber and EPDM used for outdoor insulators under accelerated ultraviolet weathering condition [J]. IEEE Transaction on Dielectrics and Electrical Insulation, 2005, 12(5): 1015-1024.
[6] JIA Z D, GAO H F, GUAN Z C, WANG L M, YANG J. Study on hydrophobicity of transfer of RTV coatings based on a modification of absorption and cohesion theory [J]. IEEE Transactions on Dielectrics and Electrical Insulation, 2006, 13(6): 1317-1324.
[7] LE Q T, PIREAUX J J, VERBIST J J. Surface modification of PET films with RF plasma and adhesion of in situ evaporated Al on PET [J]. Surface and Interface Analysis, 1994, 22(1/12): 224-229.
[8] XIE Hua-lin, TANG You-gen, LI Yu-jie, LI Li-bo. Determination of trace multi-elements in coal fly ash by inductively coupled plasma mass spectrometry [J]. Journal of Central South University of Technology, 2007, 14(1): 68-72.
[9] QU Quan-yan, QIU Wan-qi, ZENG De-chang, LIU Zhong-wu, DAI Ming-jiang, ZHOU Ke-song. Effects of deposition parameters on microstructure and thermal conductivity of diamond films deposited by DC arc plasma jet chemical vapor deposition [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(1): 131-137.
[10] MARAIS S, METAYER M, LABBE M, VALLETO J M, ALEXANDRE S, SAITER J M, PONCIN-EPAILLARD F. Surface modification by low-pressure glow discharge plasma of an unsaturated polyester resin: Effect on water diffusivity and permeability [J]. Surface and Coatings Technology, 1999, 122(2/3): 247-259.
[11] HOPKINS J, BADYAL J P S. CF4 plasma treatment of asymmetric polysulfone membranes [J]. Langmuir, 1996, 12(15): 3666-3670.
[12] HOPKINS J, BADYAL J P S. Nonequilibrium glow discharge fluorination of polymer surfaces [J]. The Journal of Physical Chemistry, 1995, 99(12): 4261-4264.
[13] COULSON S R, WOODWARD I S, BREWER S A, WILLIS C, BADYAL J P S. Ultralow surface energy plasma polymer films [J]. Chemistry of Materials, 2000, 12(7): 2031-2038.
[14] RYAN M E, FONSECA J L C, TASKER S, BADYAL J P S. Plasma polymerization of sputtered poly(tetrafluoroethylene) [J]. The Journal of Physical Chemistry, 1995, 99(18): 7060-7064.
[15] XIAO Jian-rong, XU Hui, WANG Huan-you, MA Song-shan. Effects of annealing on structural and electric property of fluorinated diamond-like carbon thin films [J]. Journal of Central South University: Science and Technology, 2007, 38(4): 669-673. (in Chinese)
[16] POPAT R H, SUTHERLAND I, SHANG E S. Vapour-phase chemical derivatisation for the determination of surface functional groups by X-ray photoelectron spectroscopy [J]. Journal of Materials Chemistry, 1995, 5(5): 713-717.
[17] RYAN M E, BADYAL J P S. Surface texturing of PTFE film using nonequilibrium plasmas [J]. Macromolecules, 1995, 28(5): 1377-1382.
[18] GODFTEY S P, KINMOND E J, BADYAL J P S. Plasmachemical functionalization of porous polystyrene beads [J]. Chemistry of Materials, 2001, 13(2): 513-518.
[19] WOODWARD I, SCHOFIELD W C E, ROUCOULES V, BADYAL J P S. Super-hydrophobic surfaces produced by plasma fluorination of polybutadiene films [J]. Langmuir, 2003, 19(8): 3432-3438.
[20] RIEKERINK M B O, TERLINGEN J G A, ENGBERS G H M, FEIJEN J. Selective etching of semicrystalline polymers: CF4 gas plasma treatment of poly(ethylene) [J]. Langmuir, 1999, 15(14): 4847-4856.
[21] ANAND M, COHEN R E, BADDOUR R F. Surface modification of low density polyethylene in a fluorine gas plasma [J]. Polymer, 1981, 22(3): 361-371.
[22] YAN Y H, CHAN-PARK M B, YUE C Y. CF4 Plasma treatment of poly(dimethylsiloxane) effect of fillers and its application to high-aspect-ratio UV embossing [J]. Langmuir, 2005, 21(19): 8905-8912.
[23] PATEL N M, DWIGHT D W, HEDRICK J L, WEBSTER D C, MCGRATH J E. Surface and bulk phase separation in block copolymers and their blends. Polysulfone/polysiloxane [J]. Macromolecules, 1988, 21(9): 2689-2696.
[24] SCHABEL M J, PETERSON T W, MUSCAT A J. Macromolecule formation in low density CF4 plasmas: The influence of H2 [J]. Journal of Applied Physics, 2003, 93(3): 1389-1402.
(Edited by YANG You-ping)
Foundation item: Project(05JT1034) supported by the Plan of Science and Technology Bureau of Hunan Province, China
Received date: 2008-11-07; Accepted date: 2009-02-14
Corresponding author: GAO Song-hua, PhD; Tel: +86-731-5641957; E-mail: gaosonghua2005@126.com