
Corrosion characteristics of copper in magnetized sea water
ZHANG Peng(张鹏)1, GUO Bin(郭斌)1, JIN Yong-ping(金永平)1, CHENG Shu-kang(程树康)2
1. School of Materials Science and Engineering, Harbin Institute of Technology, Harbin 150001, China;
2. Institute of Electromagnetic and Electronic Technology, Harbin Institute of Technology, Harbin 150001, China
Received 15 July 2007; accepted 10 September 2007
Abstract: The corrosion characteristics of copper in magnetic action system were investigated by mass loss method, electrochemical test, scanning electron microscopy (SEM) and energy analysis. It is found that the corrosion process of copper is influenced by magnetic field. The flow corrosion rate of copper decreases at the initial segment, then drives to gentle stage at the final segment. From electrochemical test, the corrosion rate of copper in the magnetized sea water is minimal compared with that in 3.5% NaCl solution and sea water. Electrochemical impedance spectroscopy (EIS) plots of copper in 3.5% NaCl, sea water and magnetized sea water are similar. However, EIS plot of copper in magnetized sea water shifts rightwards due to the effect of magnetic field on sea water. The corrosion process of copper in magnetized sea water is pitting corrosion. The surfaces of samples are finer in magnetized sea water relative to those in 3.5% NaCl solution and sea water. The corrosion products of copper include large amount of Cu element, O element and Cl element. Cu2O and CuCl2 are the primary products. This suggests that electromagnetic treatment has remarkable effect on the corrosion of copper.
Key words: copper; corrosion; magnetization; sea water
1 Introduction
Sea water system is the cooling system of ship crafts and factories near sea, and also is the operating system of oil-fields and fresh-water factories [1-2]. The cooling systems of some electric machines and rotary magnetic equipments have the effect of magnetic field. According to Faraday’s law of electromagnetic induction, electric filed can be produced when the conducting fluid is influenced by magnetic field. The flow pattern and the performance of sea water can be influenced by the interaction of electric filed and magnetic field [3]. Due to its high thermal conductivity, anticorrosion and antifouling properties, copper is frequently used in heat exchangers, heat conductors and marine engineering [4-6]. Many accidents of copper corrosion have happened in the cooling system of electric machine recently. And the pitting corrosions of copper pipes are also serious problems [7]. Until now, researches on the effects of magnetic field on copper corrosion have been reported scarcely [8-9]. So the corrosion mechanism of copper in magnetized sea water should be investigated.
Electrochemical corrosion is the result of the electrode reactions of corrosion batteries. Polarization curve shows the relationship between polarization value of corrosion electrode and polarization current density. Electrochemical impedance spectroscopy (EIS) is an effective method to study electrochemical corrosion process and electrode surface state [10]. In this study, the flow corrosion process of copper in the magnetic action system was investigated by using mass loss method, the electrochemical corrosion processes of copper in neutral 3.5% NaCl solution, sea water and magnetized sea water were investigated by electrochemical test, scanning electron microscopy (SEM) and energy spectrum analysis, and the corrosion characteristics of copper in magnetized sea water were analyzed.
2 Experimental
2.1 Experimental material and medium
Experimental material was copper. The dimensions of samples were 50 mm×25 mm×2 mm. The chemical constitutions(mass fraction, %) of copper were: 99.9 Cu, 0.005 Fe, 0.005 Pb, 0.005 S, 0.06 O, 0.14 Sn, 0.002 As, 0.002 Sb, 0.002 Ni, 0.002 Bi, 0.005 Zn.
The electrochemical samples were sanded to 800# with waterproof abrasive paper, then defatted, cleaned and dried. The mass less samples were sanded to 600#, then defatted, cleaned and dried. Experimental media were neutral 3.5% NaCl solution, artificial seawater that was deployed with marine salt and magnetized sea water that was magnetized for 10 d by magnetic field (magnetic intensity is 0.2 T).
2.2 Experiment equipment
Fig.1 shows the flow chart of flow corrosion experiment. The magnetic intensity is 0.2 T, the frequency is 150 Hz, the bulk of tank is 200 L, and the flow rate is 0.5 m3/h. The artificial sea water was injected into the tank, entered into the conduit with the pump and influenced by the electromagnetic field, and then looped back to the tank.
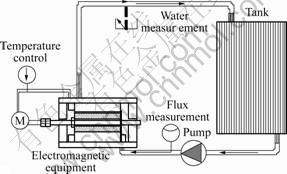
Fig.1 Flow chart of flow corrosion experiment
2.3 Measurement of flow corrosion rate
Mass loss method can be used to measure the flow corrosion rate. The computed equation of massless rate can be expressed as
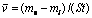 (1)
(1)
where  is the average corrosion rate, g/(m2?h); mo is the original mass of samples, g; mi is the mass of samples after removing the corrosion products, g; S is the naked area of samples, m2; t is the corrosion time, h.
is the average corrosion rate, g/(m2?h); mo is the original mass of samples, g; mi is the mass of samples after removing the corrosion products, g; S is the naked area of samples, m2; t is the corrosion time, h.
The dimensions of samples were measured by square caliper which was accurated to 0.1 μm. And the samples were weighed by analytical balance with accuracy of 0.1 mg. The corrosion products of samples can be removed by electrochemical striping method.
2.4 Electrochemical corrosion test
The electrochemical work station CHI604C was used. Test system is a classical three-electrode system, investigative electrode is the corrosion sample, aided electrode is the platinized titanium electrode and reference electrode is the saturated calomel electrode. The scanning velocity of polarization curve is 5 mV/s. The variation range of voltage is from -1.5 to 1.5 V. The frequency range of alternating current impedance is from 1 Hz to 10 kHz. The EIS can be measured from high frequency and the delay time is 2 s. The superimposed alternating voltage is 5 mV. The temperature is (25±1)℃.
3 Results and discussion
3.1 Flow corrosion rate
Fig.2 shows the flow corrosion rate of copper in flow corrosion experiment. It can be observed that the flow corrosion rate of copper decreases at the initial segment, and then drives to gentle stage at the final segment.
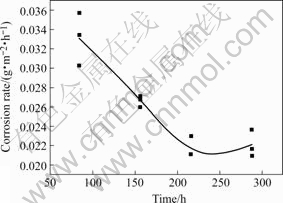
Fig.2 Flow corrosion rate of copper
3.2 Flow corrosion morphologies
Fig.3 shows surface morphologies of copper samples in magnetic system at different times. It can be found that the corrosion process of copper is pitting corrosion obviously. The sample corroded for 3 d is uniform corrosion, and the corrosion products deposit on the surface. However, the protective film is formed on the surface of samples corroded for 6 d, owing to continuous deposition of corrosion products. So, the pitting corrosion is restrained. But the copper is dissolved and the pit depth increases during the corrosion process, which can be seen in Figs.3 (a) and (b).
3.3 Polarization curve
Fig.4 shows the polarization curve of copper in different media. According to the corrosion dynamic equations under the polarization control, the corrosion potential and the corrosion current can be calculated by Tafel linear extrapolation, which is listed in Table 1. It is obviously found that the corrosion rate of copper in magnetized sea water is minimal.
3.4 Electrochemical impedance spectroscopy
Figs.5 and 6 show the Nyquist plot and Bode diagrams of copper samples in three media, respectively. It can be observed that EIS plots of copper are similar, which have two times constant, including capacitive reactance arc of low-frequency zone and inductive reactance arc of high-frequency zone. EIS plot of copper in magnetized sea water shifts rightwards, attributed to the effect of magnetic field on sea water. According to CAO’s theories [11], Faraday impedance of anode can be reflected by EIS plot. The velocity of Faraday process can be controlled by electrode potential and another state variable that is thickness or percentage of coverage of surface film. The shrinkage of inductive character in high-frequency zone shows the electrodes have surface film. Faraday admittance can be expressed as
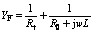 (2)
(2)
where Rt is the interface reaction electric resistance in active regions; R0 is the equivalent resistance related to solution or growth of film in pitting corrosion active spots; L is the equivalent induction related to thickness or percentage of coverage of surface film in active spots. So, Faraday impedance is parallel connection of electric charge transfer resistance Rt and the compound component that is connection in series of equivalent resistance R0 and equivalent induction. The equivalent circuit of electrode system can be written as R(QR(RL)), which can be obtained by the connection in series of solution resistance R1 and compound component that is parallel connection of Faraday impedance and interface equivalent drain capacitance Q, as shown in Fig.7. The component Q can be defined with admittance and n.
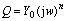 (3)
(3)
For n=0, Q represents the electric resistance; n=1, capacitance.

Fig.3 Surface morphologies of copper in magnetic system at different times: (a) 3 d; (b) 6 d; (c) 9 d; (d) 12 d
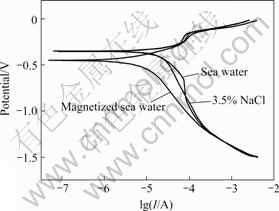
Fig.4 Polarization curves of copper in various media
Table 1 Corrosion potential and current of copper

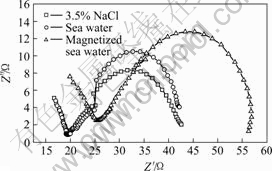
Fig.5 Nyquist plots of copper in various media
Fig.6 Bode diagram of copper in various media: (a) Dual- logarithm graph of real component of impedance as frequency; (b) Relationship between phase and logarithm of frequency
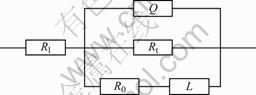
Fig.7 Equivalent circuit of corrosion system
3.5 Electrochemical corrosion morphologies
Fig.8 shows the surface morphologies of electrochemical corrosion samples in various media. It can be seen that uniform corrosion takes place in neutral 3.5% NaCl solution; loose pores appear in sea water and basal body are naked; the samples in magnetized sea water are compact, only a few pores exist. So, anticorrosion effect is obvious.
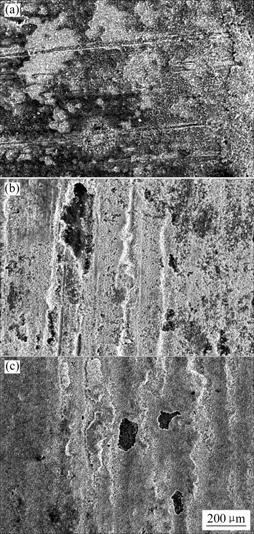
Fig.8 Surface morphologies of copper in various media: (a) 3.5% NaCl solution; (b) Sea water; (c) Magnetized sea water
3.6 Composition analysis
Fig.9 shows the components of electrochemical copper samples. It can be observed that large amount of O element, Cl element and Cu element exists on the surface of samples. The corrosion products in 3.5% NaCl solution include Cu element, O element and Cl element chiefly. However, corrosion products in sea water and magnetized sea water mainly contain Cu element and Cl element.
3.7 Analysis of corrosion process
According to EIS characters, corrosion morphology, energy analysis and electrochemical corrosion theories of copper samples, the corrosion process is mainly pitting corrosion that can be described as follows.
Stage Ⅰ, copper corrodes generally in the solution according to the equations [12-13].
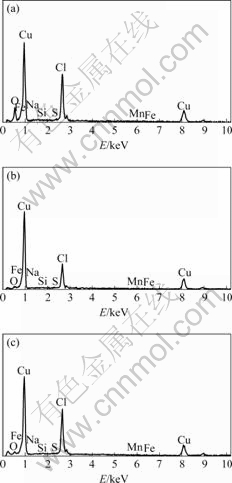
Fig.9 Surface morphologies of copper in various media: (a) 3.5% NaCl solution; (b) Sea water; (c) Magnetized sea water
Cu+OH-→Cu(OH)(ads)+e+ (4)
2Cu+OH(ads) Cu2O+H2O (5)
Cu2O+H2O (5)
Cu(OH)(ads)+Cl-→CuCl(ads)+OH- (6)
CuCl(ads)+Cl-→CuCl2 (7)
So, the corrosion products in neutral 3.5% NaCl solution are Cu2O and CuCl chiefly, but the corrosion products in sea water and magnetized sea water are mainly CuCl and CuCl2.
Stage Ⅱ, protective film is produced due to continuous deposition of the corrosion products, which separates the corrosion zone, so pitting corrosion is restrained. Physico-chemical performance of sea water is changed by electromagnetic treatment, and the solution is of better capability for dissolved gas. It can be found that the capability of dissolved oxygen increases by 25.2% in the experiment. The dissolved oxygen of sea water is the important factor of sea corrosion because sea corrosion of many metals is controlled by oxygen depolarization.
Stage Ⅲ, copper in the pore continues dissolving and the depth of pore increases. During the corrosion process, it can be observed that the color of medium becomes green due to dissolution of corrosion surface, which indicates that the basal body is corroded continually.
4 Conclusions
1) The flow corrosion rate of copper decreases at the initial segment, then drives to gentle stage at the final segment. The corrosion process of copper in magnetized sea water is pitting corrosion.
2) The corrosion rate of copper in magnetized sea water is minimal. EIS plot of copper in magnetized sea water shifts rightwards due to the effect of magnetic field on sea water.
3) The electrochemical samples in magnetized sea water are compact, only a few pores appear, anticorrosion effect is obvious. The corrosion products in neutral 3.5% NaCl solution are Cu2O and CuCl chiefly, but the corrosion products in sea water and magnetized sea water are mainly CuCl and CuCl2.
References
[1] PHILIP L. A new concept in marine desalination—The thermal compression distillation plant [J]. Marine Technology, 1990, 27(3): 135-157.
[2] DREIZIN Y. A shkelon seawater desalination project-off-taker’s self costs, supplied water costs, total costs and benefits [J]. Desalination, 2006, 190: 104-116.
[3] TACKEN R A, JANSSEN L J J. Applications of magnetoelectrolysis [J]. J Appl Electrochem, 1995, 25: 1-5.
[4] SCHUMACHER M. Seawater corrosion handbook [M]. New Jersey: Park Ridge, 1979: 98-104.
[5] GLOVER T G. Copper-nickel alloy for the construction of ship and boat hulls [J]. British Corrosion Journal, 1982, 17(4): 155-158.
[6] AGARWAL D C. Effect of ammoniacal sea water on material properties of copper-nickel alloy [J]. British Corrosion Journal, 2002, 37(2): 105-110.
[7] ZHAO Yue-hong, LIN Le-yun, CUI Da-wei. Localized corrosion of copper alloys in China sea water for 16 years [J]. Trans Nonferrous Met Soc China, 2004, 14(6): 1082-1090.
[8] LIU Wei-guo, LIU Jian-dong, ZHAO Chen-long. Corrosion prevention mechanism in magnetic treatment of water [J]. J Chin Soc Corros Prot, 2006, 27(4): 196-198. (in Chinese)
[9] GU Z H, CHEN J, FAHIDY T Z. The magnetic field effect on the preoscillatory formation kinetics of anodic oxide layers [J]. Electrochim Acta, 1993, 18(17): 2631-2634.
[10] LEI Yong-quan, WU Yu-ming, YANG Quan-ming, WU Jing, WANG Qi-dong. Electrochemical behaviour of some mechanically alloyed Mg-Ni-based amorphous hydrogen storage alloys [J]. Z Phys Chem Bd, 1994, 183: 379-384.
[11] CAO C N, ZHANG J Q. An introduction to electrochemical impedance spectroscopy [M]. Beijing: Science Press, 2002: 84. (in Chinese)
[12] BARCIA O E, MATTOS O R, PEBERE N. Mass-transport study for the electrodissolution of copper in IM hydrochloric acid solution by impedance [J]. J Electrochem Soc, 1993, 140(10): 2825-2833.
[13] CRUNDWELL F K. Anodic dissolution of copper in hydrochloric acid solutions [J]. Electrocim Acta, 1992, 37(15): 2707-2710.
Corresponding author: ZHANG Peng; Tel: +86-451-86402775; E-mail: zhangpeng910@yahoo.com.cn
(Edited by CHEN Wei-ping)