氢氧化钠分解钼酸铅矿的热力学分析
张 刚,赵中伟,李江涛, 陈爱良,霍广生,李洪桂
(中南大学 冶金科学与工程学院,湖南 长沙,410083)
摘 要:针对钼酸铅矿的氢氧化钠分解过程,根据电荷平衡和质量平衡的原理,运用热力学数据绘制25 ℃时Pb-Mo-H2O系组分的浓度对数-pH图。利用热力学平衡图对氢氧化钠分解钼酸铅矿的工艺条件进行讨论。研究结果表明:整个pH值范围内可分为3个物质稳定区,即pH<6.17时为H2MoO4的稳定区;6.17<pH<11时为钼酸铅的稳定区;pH>11时为Pb(OH)2的稳定区,在该范围内随着pH值升高,当Pb(OH)2达到过饱和时,溶液中开始析出Pb(OH)2沉淀,从而实现钼酸铅的碱分解过程。通过对Pb-Mo-H2O系的热力学分析,苛性钠分解彩钼铅矿在一定的pH值下是可行的,但在碱分解过程中Pb2+与羟基形成了配合物,主要是以 的形式存在而大量进入溶液,因此,尚需进一步除铅。
的形式存在而大量进入溶液,因此,尚需进一步除铅。
关键词:钼酸铅矿;热力学;浓度对数-pH图;氢氧化钠
中图分类号:TF111.31 文献标识码:A 文章编号:1672-7207(2008)05-0902-05
Thermodynamics analysis on sodium hydroxide decomposition of wulfenite
ZHANG Gang, ZHAO Zhong-wei, LI Jiang-tao, CHEN Ai-liang, HUO Guang-sheng, LI Hong-gui
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: According to the law of charge balance equilibrium and the law of conservation of mass, the logarithm concentration-pH diagram of Pb-Mo-H2O system was established on the basis of thermodynamic data at 25 ℃. Thermodynamic analysis was carried out to show the effect of technical conditions on sodium hydroxide decomposition of wulfenite. The results show that the whole pH value of the system is divided into three areas. The first one is the stable area of H2MoO4, which is located in the range of the pH value less than 6.17. The second area is the stable area of PbMoO4, which is located at the pH value from 6.17 to 11. The third one is located in the range of the pH value higher than 11, which is the stable area of Pb(OH)2. In the third area, Pb(OH)2 is gradually supersaturated in solution with the increase of pH value, so the Pb(OH)2 is deposited as sediment. As a result, the processing of wulfenite leaching by sodium hydroxide is carried out. Additionally, feasibility of the processing of wulfenite leaching by sodium hydroxide is proved, but Pb2+ reacts with hydroxyl to form complexes which is leached into solutions. So removing the complexes lead from solution is needed.
Key words: wulfenite; thermodynamics; logarithm concentration-pH diagram; sodium hydroxide
钼是现代工业中重要的战略物资之一,主要用作高强耐蚀合金钢的合金元素;金属钼则用于核反应堆,喷气发动机、火箭发射器等。此外,在化工和石油等许多领域也有着广泛的应用。随着工业经济的发展,尤其是国际钢铁业的发展,对钼的市场需求量不断增加,致使近年来金属钼及其化工产品的价格与之前相比大幅度上升。因此,对于钼资源的研究开发越来越受到各国科研机构及企业的重视[1-2]。
钼在自然界中主要以辉钼矿MoS2的形式赋存,除此之外还存在多种与钼共生的多元素氧化矿,其中具有工业价值的有钼酸钙矿(CaMoO4)、钼酸铁矿(Fe2O3?MoO3?7H2O)和钼酸铅(PbMoO4)。彩钼铅矿(PbMoO4)即钼酸铅矿作为钼资源的一种,常产于铅矿床的氧化带。美国的科罗拉多以及墨西哥和智利,还有在我国的湖南、云南等地均发现该种矿床,它易于开采,可以同时回收钼和铅2种元素,有较好的经济效益[3-4]。在处理工艺方面,为了从彩钼铅矿中回收有价金属,矿石需首先通过重选得到粗精矿,然后,经过湿法工艺回收矿石中的钼,同时分离除铅。通常使用的浸出剂有硫化钠、氢氧化钠、硝酸、盐酸、硫酸和碳酸钠等,其中比较常用的工艺有钼酸铅矿的硫化钠浸出和氢氧化钠浸出。但目前关于钼酸铅矿处理方面的研究报道基本都处于工艺研究阶段,对其浸出过程的理论问题研究报道很少,限制了人们对于浸出反应的深层理解,使得对工艺研究带有一定程度的盲 目性[5]。在此,本文作者对氢氧化钠分解彩钼铅矿工艺过程进行热力学分析,通过研究Pb-Mo-H2O体系的溶解平衡,根据电荷平衡和质量平衡的原理,并运用已有的热力学数据和热力学计算方法绘制25 ℃时Pb-Mo-H2O体系的浓度对数-pH图。
1 热力学数据及计算
1.1 热力学数据
计算所用到的热力学数据及平衡反应方程式见表1。从表1提供的平衡关系式可知,在不同pH值条件下,有不同的沉淀物质生成。不考虑钼的各种同多酸,当pH值较低时,体系中 转变为H2MoO4沉淀,铅则以Pb2+形式存在;当pH值较高时,Pb2+转变为Pb(OH)2沉淀。以此作为限定条件,对该体系进行热力学分析计算,由于缺乏相关物质的活度系数,在本文计算过程中以物质的浓度代替活度。
转变为H2MoO4沉淀,铅则以Pb2+形式存在;当pH值较高时,Pb2+转变为Pb(OH)2沉淀。以此作为限定条件,对该体系进行热力学分析计算,由于缺乏相关物质的活度系数,在本文计算过程中以物质的浓度代替活度。
表1 Pb-Mo-H2O系平衡方程式及平衡常数(25 ℃)[6]
Table 1 Equilibrium equation and Equilibrium constant of Pb-Mo-H2O system (25 ℃)

1.2 热力学计算
根据电荷平衡和质量平衡的原理[7-15],可得:


在低pH值范围内,发生如下反应:
PbMoO4(s)+2H+=Pb2++H2MoO4(s),
即PbMoO4转化为H2MoO4,体系处于H2MoO4的稳定区,根据表1中反应式(10)和(11)可得:
 。 (14)
。 (14)
当体系处于PbMoO4的稳定区时,可得:
 。 (15)
。 (15)
在高pH值范围内,发生如下反应:
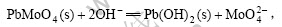
即体系在较高pH值条件下,PbMoO4将转化为Pb(OH)2,体系处于Pb(OH)2的稳定区,根据表1中反应式(2)和(3)可得:
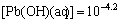 。 (16)
。 (16)
此外,体系中所有pH值范围内都满足:
 。 (17)
。 (17)
以上方程式中,未知的有[Pb2+], ,[Mo]T和[Pb]T 4个变量,可由式(1),(6),(15)和(17)联立方程组求解。
,[Mo]T和[Pb]T 4个变量,可由式(1),(6),(15)和(17)联立方程组求解。
根据计算结果,绘制出25 ℃时的Pb-Mo-H2O浓度对数-pH图,见图1。

图1 25 ℃时Pb-Mo-H2O体系lg c-pH图
Fig.1 Logarithm concentration-pH diagram of Pb-Mo-H2O system at 25 ℃
2 结果讨论
从图1可以看到,Pb-Mo-H2O系在整个pH值范围内以pH值为6.17和11为界可划分为3个区域,以垂直虚线a和b表示。
在虚线a与b之间,溶液中所有离子基团都由 的溶解平衡所制约,其溶度积在所有pH值范围内为一定值,即[Pb2+]
的溶解平衡所制约,其溶度积在所有pH值范围内为一定值,即[Pb2+] =10-13。随着溶液pH值的上升,OH-浓度逐渐增加,其与Pb2+的结合能力也逐渐增强,因而图1中铅的各种羟基配合物浓度不断增加,而游离的Pb2+离子浓度则受到强烈抑制而不断下降。由于
=10-13。随着溶液pH值的上升,OH-浓度逐渐增加,其与Pb2+的结合能力也逐渐增强,因而图1中铅的各种羟基配合物浓度不断增加,而游离的Pb2+离子浓度则受到强烈抑制而不断下降。由于 的溶度积一定,游离Pb2+浓度下降,相应的
的溶度积一定,游离Pb2+浓度下降,相应的 浓度则迅速上升。其他各种含钼离子基团的浓度变化则与
浓度则迅速上升。其他各种含钼离子基团的浓度变化则与 离子之间的平衡而变化。而且由于pH值增加时OH-活度增加,当可溶性Pb(OH)2浓度恒定时,游离Pb2+浓度必定迅速下降,如图1中Pb2+浓度曲线所示;而由于上面提到的
离子之间的平衡而变化。而且由于pH值增加时OH-活度增加,当可溶性Pb(OH)2浓度恒定时,游离Pb2+浓度必定迅速下降,如图1中Pb2+浓度曲线所示;而由于上面提到的 溶解平衡的制约关系,相应地
溶解平衡的制约关系,相应地 浓度则会迅速增加,并且随着溶液的碱性增强,钼的平衡浓度越高;当溶液pH值高于图1中虚线b所处的pH值时,可溶性Pb(OH)2的浓度不再恒定,而是呈不断增加的趋势,当其浓度达到过饱和时,溶液中开始析出Pb(OH)2的固体,也就是说,
浓度则会迅速增加,并且随着溶液的碱性增强,钼的平衡浓度越高;当溶液pH值高于图1中虚线b所处的pH值时,可溶性Pb(OH)2的浓度不再恒定,而是呈不断增加的趋势,当其浓度达到过饱和时,溶液中开始析出Pb(OH)2的固体,也就是说, 将不断转变成Pb(OH)2固体和
将不断转变成Pb(OH)2固体和 而被浸出,在工业上称为碱性浸出,其反应为PbMoO4(s)+2OH-=Pb(OH)2(s)+
而被浸出,在工业上称为碱性浸出,其反应为PbMoO4(s)+2OH-=Pb(OH)2(s)+  ,Pb(OH)2在此区域内为惟一稳定固相物质。与此同时,由图2可以看出,在虚线b的右边,由于部分铅转化为沉淀,溶液中总铅浓度[Pb]T与总钼浓度[Mo]T不再相等,铅浓度要低得多;但值得注意的是,尽管如此,总铅浓度[Pb]T的变化趋势仍然是随着溶液pH值的升高而增加,其原因是铅离子与氢氧根生成了各种羟基配合物而进入溶液,在这些配合物中主要是
,Pb(OH)2在此区域内为惟一稳定固相物质。与此同时,由图2可以看出,在虚线b的右边,由于部分铅转化为沉淀,溶液中总铅浓度[Pb]T与总钼浓度[Mo]T不再相等,铅浓度要低得多;但值得注意的是,尽管如此,总铅浓度[Pb]T的变化趋势仍然是随着溶液pH值的升高而增加,其原因是铅离子与氢氧根生成了各种羟基配合物而进入溶液,在这些配合物中主要是 的浓度随pH值增加而不断增大,造成总铅浓度上升。
的浓度随pH值增加而不断增大,造成总铅浓度上升。

图2 25 ℃时Pb-Mo-H2O体系中[Pb]T与[Mo]T浓度对数-pH图
Fig.2 Logarithm concentration-pH diagram of [Pb]T and [Mo]T of Pb-Mo-H2O system at 25 ℃
H2MoO4(aq)的浓度在虚线a和b之间的变化趋势是随着pH值的降低而逐步上升。由于pH值下降时,H+活度增加,与钼酸根离子的结合趋势增强。当pH值下降到相当于a线左右的pH值时,H2MoO4(aq)恰恰达到饱和,再进一步降低pH值,低于a线所代表的pH值时,H2MoO4(aq)将会因过饱和而析出H2MoO4沉淀。与前面分析铅的情况相类似,这会导致 浓度的迅速下降和Pb2+浓度上升。反映在图2中,出现了总铅的浓度大于总钼浓度,这实际相当于PbMoO4的酸性浸出,即PbMoO4(s)+2H+=Pb2++H2MoO4(s),该区域中H2MoO4是惟一稳定固相物质。需要指出的是,以上计算是忽略了钼离子在酸性条件下聚合生成同多酸反应的情况下得到的,否则,钼的平衡浓度应更高些。此外,图2中低pH值下总钼浓度有些上升。这是由于溶液在比较高的酸度时,钼则倾向于形成钼酰阳离子,如图1中
浓度的迅速下降和Pb2+浓度上升。反映在图2中,出现了总铅的浓度大于总钼浓度,这实际相当于PbMoO4的酸性浸出,即PbMoO4(s)+2H+=Pb2++H2MoO4(s),该区域中H2MoO4是惟一稳定固相物质。需要指出的是,以上计算是忽略了钼离子在酸性条件下聚合生成同多酸反应的情况下得到的,否则,钼的平衡浓度应更高些。此外,图2中低pH值下总钼浓度有些上升。这是由于溶液在比较高的酸度时,钼则倾向于形成钼酰阳离子,如图1中 的浓度曲线随pH值下降而不断上升,造成
的浓度曲线随pH值下降而不断上升,造成 升高。
升高。
以上分析结果与文献研究结果是吻合的,并可以用于解释实验现象。如文献[16]中报道了氢氧化钠处理含Mo 13.48%,含Pb 40.1%的彩钼铅矿,当氢氧化钠用量为理论用量的4.3倍、温度为80 ℃、浸出120 min、液固比为4?1时,Mo的浸出率达到95.3%,但Pb也有50%浸出;根据图1可知,铅的浸出主要是由于高pH值条件下,铅离子与羟基生成多种羟基配合物进入溶液。由于有较多的铅存在于浸出液,故在后续的冶炼操作中pH回调时,就会因Pb2+浓度上升,而抑制 浓度,造成
浓度,造成 与Pb2+重新结合生成钼酸铅二次沉淀而影响总回收率,在试验中发现这一情况后,采取2种方法降低浸出液中的可溶性铅。一种是Na2S作为抑制剂,使铅形成稳定的PbS沉淀,反应如下:
与Pb2+重新结合生成钼酸铅二次沉淀而影响总回收率,在试验中发现这一情况后,采取2种方法降低浸出液中的可溶性铅。一种是Na2S作为抑制剂,使铅形成稳定的PbS沉淀,反应如下:
Na2S(l)+Pb2+=PbS(s)+2Na2+。
另一种方法是加入强氧化剂NaClO3,使铅以PbO2的形式沉淀,反应如下:
NaClO3+3Na2PbO2+3H2O→3PbO2↓+NaCl+6NaOH。
若在浸出时直接采用硫化钠浸出,则浸出过程中生成难溶的PbS沉淀而降低溶液游离Pb2+浓度,促使PbMoO4分解而转化为 得以浸出。如在文献[17-18]中采用Na2S·9H2O为矿量的50%,温度为 80 ℃,浸出时间为1 h,液固比为5?1及两级逆流浸出的条件下,钼的浸出率达到95.1%,铅的回收率为94.9%,取得了较好的效果。
得以浸出。如在文献[17-18]中采用Na2S·9H2O为矿量的50%,温度为 80 ℃,浸出时间为1 h,液固比为5?1及两级逆流浸出的条件下,钼的浸出率达到95.1%,铅的回收率为94.9%,取得了较好的效果。
综上所述,氢氧化钠分解彩钼铅矿是可行的,但由于在浸出过程中铅离子与氢氧根离子结合形成配合物进入溶液,因此,要实现钼与铅的彻底分离尚需除铅的工艺。
3 结 论
a. 绘制出25 ℃时Pb-Mo-H2O的浓度对数-pH图,指出整个pH值范围内可分为3个物质稳定区:pH<6.17时为H2MoO4的稳定区;6.17<pH<11时为钼酸铅的稳定区;pH>11时为Pb(OH)2的稳定区。
b. 阐述了溶液中铅与钼的各种离子基团随pH值的变化规律,指出钼酸铅在溶液pH值低于6.17时是酸性浸出,钼以H2MoO4固体形式被浸出;pH值高于11时,实现钼酸铅的碱性浸出,钼以 的形式进入溶液,而此时浸出液中的铅主要以
的形式进入溶液,而此时浸出液中的铅主要以 的形式存在。
的形式存在。
c. 苛性钠分解彩钼铅矿pH值高于11的条件下是可行的,但要彻底实现钼与铅的分离,需采用添加抑制剂Na2S或加入强氧化剂NaClO3的方法来进一步 除铅。
参考文献:
[1] 《有色金属提取冶金手册》编辑委员会. 有色金属提取冶金手册—稀有高熔点金属(上)[M]. 北京: 冶金工业出版社, 1999: 259-277.
Editorial Board of 《A Handbook For Extractive Metallurgy of Nonferrous Metals》. A handbook for extractive metallurgy of nonferrous metals—Rare refractory metals: Part 1[M]. Beijing: Metallurgical Industry Press, 1999: 259-277.
[2] 张启修, 赵秦生. 钨钼冶金学[M]. 北京: 冶金工业出版社, 2005: 42-61.
ZHANG Qi-xiu, ZHAO Qing-sheng. Metallurgy of tungsten and molybdenum[M]. Beijing: Metallurgical Industry Press, 2005: 42-61.
[3] Bideaux R A. The desert mineral wulfenite[J]. Rocks & Minerals, 1990, 66(1): 10-30.
[4] Moore T. Minerals from the San Francisco mine, Sonora, Mexico[J]. Mineralogical Record, 2004, 35(6): 41-61, 65.
[5] 程光荣. 我国钼铅矿资源及加工现状[J]. 钼业经济技术, 1988(3): 60-62.
CHENG Guang-rong. Present situation of wulfenite resource development of our country and development trend[J]. Economics and Technology of Molybdenum, 1988(3): 60-62.
[6] Sillén L G, Martell A E. Stability constant of metal-ion complex: Supplement No.1[M]. London: The Chemical Society, 1971: 128-170
[7] 王淀佐, 胡岳华. 浮选溶液化学[M]. 长沙: 湖南科学技术出版社, 1988: 198-207.
WANG Dian-zuo, HU Yue-hua. Solution chemistry of flotation[M]. Changsha: Hunan Science & Technology Press, 1988: 198-207.
[8] 赵中伟, 胡宇杰, 李洪桂. 一种用EXCEL进行冶金热力学平衡计算的新方法[J]. 稀有金属与硬质合金, 2005, 33(1): 48-51.
ZHAO Zhong-wei, HU Yu-jie, LI Hong-gui. A new method for metallurgical thermodynamic equilibrium calculation by EXCEL[J]. Rare Metals and Cemented Carbides, 2005, 33(1): 48-51.
[9] 丁治英, 赵中伟. 氟盐溶液浸出白钨矿的热力学分析[J]. 稀有金属与硬质合金, 2004, 32(1): 8-10.
DING Zhi-ying, ZHAO Zhong-wei. Thermodynamic analysis of scheelite leaching by fluoride solution[J]. Rare Metals and Cemented Carbides, 2004, 32(1): 8-10.
[10] 王识博, 赵中伟, 李洪桂. 磷酸盐浸出白钨矿的热力学分析[J]. 稀有金属与硬质合金, 2005, 33(1): 1-4.
WANG Shi-bo, ZHAO Zhong-wei, LI Hong-gui. Thermodynamic analysis on phosphate decomposition of scheelite[J]. Rare Metals and Cemented Carbides, 2005, 33(1): 1-4.
[11] 霍广生, 赵中伟, 吴保林. 钼的硫化反应热力学分析[J]. 中南工业大学学报: 自然科学版, 2001, 32(3): 259-261.
HUO Guang-sheng, ZHAO Zhong-wei, WU Bao-lin. Thermaldynamic analysis on sulfidation of molybdate[J]. Journal of Central South University of Technology: Natural Science, 2001, 32(3): 259-261.
[12] 杨建广, 唐谟堂, 杨声海, 等. Sn(Ⅳ)-Sb(Ⅲ)-NH3-NH4Cl-H2O体系热力学分析及其应用[J]. 中南大学学报: 自然科学版, 2005, 36(4): 582-586.
YANG Jian-guang, TANG Mo-tang, YANG Sheng-hai, et al. Thermodynamic analysis of Sn(Ⅳ)-Sb(Ⅲ)-NH3-NH4Cl-H2O system and its application[J]. Journal of Central South University: Science and Technology, 2005, 36(4): 582-586.
[13] 巨少华, 唐谟堂, 杨声海. 用MATLAB编程求解Zn(Ⅱ)-NH4Cl-NH3-H2O体系热力学模型[J]. 中南大学学报:自然科学版, 2005, 36(5): 821-827.
JU Shao-hua, TANG Mo-tang, YANG Sheng-hai. Thermodynamic model of Zn(Ⅱ)-NH4Cl-NH3-H2O system using MATLAB programming[J]. Journal of Central South University: Science and Technology, 2005, 36(5): 821-827.
[14] 王云燕, 彭文杰, 舒余德, 等. Bi(Ⅲ)-X(Cl-, NO3-)-H2O体系热力学平衡研究[J]. 中南工业大学学报: 自然科学版, 2001, 32(2): 139-141.
WANG Yun-yan, PENG Wen-jie, SHU Yu-de, et al. Thermodynamic equilibrium study of Bi(Ⅲ)-X(Cl-, NO3-)-H2O system[J]. Journal of Central South University of Technology: Natural and Science, 2001, 32(2): 139-141.
[15] 史海燕, 赵中伟. 苛性钠分解黑钨矿的热力学分析[J]. 中国钨业, 2006, 21(5): 24-27.
SHI Hai-yan, ZHAO Zhong-wei. Thermodynamics analysis on caustic sodium decomposition of wolframite[J]. China Tungsten Industry, 2006, 21(5): 24-27.
[16] 廖元双, 杨大锦, 鲁顺利. 钼铅矿提钼工艺研究[J]. 有色金属: 冶炼部分, 2006(4): 26-27, 35.
LIAO Yuan-shuang, YANG Da-jin, LU Shun-li. Study on molybdenum recovering from wulfenite[J]. Non-ferrous Metals: Metallurgy, 2006(4): 26-27, 35.
[17] 邹著适. 用钼铅矿制取钼酸铵的试验[J]. 广州化工, 1993, 21(2): 44-48.
ZOU Zhu-shi. Study on producing ammonium molybdate using molybdenum concentrate[J]. Guangzhou Chemistry, 1993, 21(2): 44-48.
[18] 马秀华, 程光荣, 王述吉, 等. 从彩钼铅矿制取钼酸铵[J]. 钼业经济技术, 1989(2): 20-29.
MA Xiu-hua, CHENG Guang-rong, WANG Shu-ji, et al. Study on producing ammonium molybdate using wulfenite[J]. Economics and Technology of Molybdenum, 1989(2): 20-29.
收稿日期:2007-12-22;修回日期:2008-02-14
基金项目:国家“863”计划资助项目(2006AA06Z122);湖南省国土资源厅矿产资源与合理开发利用科研专项计划资助项目(2006K06)
通信作者:赵中伟(1966-),男,河北永年人,教授,从事冶金及功能材料方面的研究;电话:0731-8830476;E-mail: zhaozw@mail.csu.edu.cn