Kinetics and mechanism of interfacial reaction in SCS-6 SiC continuous fiber-reinforced Ti-Al intermetallic matrix composites
来源期刊:中国有色金属学报(英文版)2006年第1期
论文作者:吕祥鸿 杨延清 马志军 刘翠霞 陈彦 艾云龙
文章页码:77 - 77
Key words:SCS-6 SiC fiber; Ti-Al intermetallics; interfacial reaction; kinetics; activation energy
Abstract:
Abstract: SCS-6 SiC continuous fiber-reinforced Ti-Al intermetallics-matrix composites were fabricated by HIP method and then heat-treated in vacuum under different conditions. The interfacial reaction kinetics and mechanism were studied by using SEM, EDS and XRD. The results show that the content fluctuation of reactive elements such as C, Ti and Si appears in interfacial reaction layers, and multi-layer interfacial reaction compounds form. Alloying element Nb in matrix remarkably diffuses into interfacial reaction zone and changes the activation energy for the interfacial reaction layer growth following a role of parabolic rate. The activation energy (Qk) and (k0) of SCS-6 SiC/super α2 and SCS-6 SiC/Ti2AlNb are 317.664 kJ/mol, 175.709 kJ/mol and 5.4438×10-2 m/s1/2, 1.44×10-5 m/s1/2; respectively, and the diffusion coefficient (DC) is about 10-18—10-20 m2/s. It is confirmed that the SCS-6 SiC/Ti-Al intermetallic composites have higher interface compatibility and stability. Furthermore, compared with SCS-6 SiC/super α2, the interface compatibility and stability of SCS-6 SiC/Ti2AlNb are even higher.
基金信息:the National Natural Science Foundation of China
the State Education Ministry Doctoral Foundation of China
the Foundation of Aviation Science of China
the Materials Engineering Center Foundation of Jiangxi Province, China

L? Xiang-hong(吕祥鸿)1, YANG Yan-qing(杨延清)1, MA Zhi-jun(马志军)1, LIU Cui-xia(刘翠霞)1,
CHEN Yan(陈 彦)1, AI Yun-long(艾云龙)2
1. State Key Laboratory of Solidification Processing, Northwestern Polytechnical University,
Xi’an 710072, China;
2. College of Materials Engineering, Nanchang Institute of Aero-technology,
Nanchang 330034, China
Received 25 April 2005; accepted 4 July 2005
Abstract: SCS-6 SiC continuous fiber-reinforced Ti-Al intermetallics-matrix composites were fabricated by HIP method and then heat-treated in vacuum under different conditions. The interfacial reaction kinetics and mechanism were studied by using SEM, EDS and XRD. The results show that the content fluctuation of reactive elements such as C, Ti and Si appears in interfacial reaction layers, and multi-layer interfacial reaction compounds form. Alloying element Nb in matrix remarkably diffuses into interfacial reaction zone and changes the activation energy for the interfacial reaction layer growth following a role of parabolic rate. The activation energy (Qk) and (k0) of SCS-6 SiC/super α2 and SCS-6 SiC/Ti2AlNb are 317.664 kJ/mol, 175.709 kJ/mol and 5.4438×10-2 m/s1/2, 1.44×10-5 m/s1/2; respectively, and the diffusion coefficient (DC) is about 10-18—10-20 m2/s. It is confirmed that the SCS-6 SiC/Ti-Al intermetallic composites have higher interface compatibility and stability. Furthermore, compared with SCS-6 SiC/super α2, the interface compatibility and stability of SCS-6 SiC/Ti2AlNb are even higher.
Key words: SCS-6 SiC fiber; Ti-Al intermetallics; interfacial reaction; kinetics; activation energy
1 Introduction
Due to its high specific strength and specific modulus, SiC fiber reinforced Ti alloy-matrix composites (TMCs) can be used for aero-engines[1]. However, the oxidation resistance of TMCs descends remarkably as soon as the temperature exceeds 600 ℃[2]. Ti-Al intermetallic compounds, with high strength, low density and excellent oxidation resistance at much higher temperature, are more suitable for the matrix of the composites[3].
It is known that the interfacial reaction exists between SiC fiber and matrix not only in the TMCs but also in the Ti-Al intermetallic compound-matrix composites reinforced by the SiC fiber (IMCs). The interfacial reaction taking place during the composite consolidation and high temperature service leads to the formation of some brittle compounds distributed in several layers at the interface. The brittle interfacial reaction products become the crack origination that makes the interface lose its stability and the ability of transfering the applied load[4].
In order to study the reaction kinetics and mechanism between SiC fiber and Ti-Al intermetallic compounds, super α2 (Ti-25Al- 10Nb-3V-1Mo) and Ti2AlNb (Ti-23Al-25Nb) were used as the matrix, which were reinforced by SCS-6 SiC continuous fiber. These composites were fabricated and heat treateded under different conditions, and the morphology and elemental distributions as well as components of interfacial reaction zone were analyzed with the help of SEM, EDS and XRD. The airm of the investigation is to forecast the reactivity of reinforce/matrix interface as well as the serving condition of IMCs.
2 Experimental
The fabricating program of SCS-6 SiC/ super α2 and SCS-6 SiC/Ti2AlNb composites was that the fiber was coated with the matrix super α2 (Ti-25Al- 10Nb-3V-1Mo) or Ti2AlNb (Ti-23Al-25Nb) alloys deposited by magnetron sputtering at first, then they were processed through hot isostatic pressing (HIP) to full density. After its fabrication, the composites were heat treated in vacuum at 700 and 800 ℃ for 1 000, 1 500 and 2 000 h respectively, in addition the latter composite was also heat treated at 900 ℃ for 50, 200 and 500 h, respectively.
Samples were cut from composites perpendicular to the fiber direction with a low- speed diamond saw. The cross section was prepared by grinding step by step with 400, 600, 1000 and 1200 grit silicon carbide metallographic papers, polishing with 1 ?m diamond paste, then etching for about 2 s with a solution made of 1 mL HF, 3 mL HNO3 and 7 mL H2O, and cleaning with water immediately. After sipping up water with filter paper, the samples were immerged in acetone or absolute alcohol for 3-5 min to get out of water. Finally the dehydrated samples were dried with hot air, encased with qualitative filter paper and put into an airer for laster use. The morphologies of interfacial reaction products were examined with JSM 6460 scanning electron microscope(SEM).The phases in the composites were identified by Panalytical X’Pert PRO X-ray diffractometer. And the elements content was analyzed with Oxford INCA7574 energy dispersive spectrometer (EDS).
3 Results and discussion
3.1 Interfacial reaction of two composites
Figs.1 and 2 show the interfacial reaction zone morphologies of SCS-6 SiC/super α2 and SCS-6 SiC/Ti2AlNb, respectively. At the course of fabrication and heat-treatment of the composites, the interfacial reaction zone becomes thicker and thicker along with the time and temperature, and the C-rich coating is gradually consumed. At the same time, a multi-layered interface reaction zone can be observed clearly in Fig.2(d). By comparing Fig.1 with Fig.2, it is clear that the interfacial reaction zone of SCS-6 SiC/Ti2AlNb is much thinner than that of SCS-6 SiC/super α2 under the same condition of heat treatment.
Fig.3 shows the EDS analytical results of interfacial reaction products of SCS-6 SiC/super α2 and SCS-6 SiC/Ti2AlNb. It can be seen from Fig.3 that the content fluctuation of reactive elements such as C, Si, Ti, Al and Nb exists in the reaction layers of the two composites, which indicates that the Si and C atoms diffuse from SCS-6 SiC fiber to the Ti-Al intermetallic compounds matrix, and the Ti, Al and Nb atoms diffuse in the opposite direction. Fig.4 shows the X-ray diffraction patterns of the two composites, indicating the phases exist in the composites. Obviously, the phases TiC, Ti5Si3 Ti3AlC and Ti3SiC2 are the interfacial reaction products. As mentioned above, the interfacial reaction products are distributed in layers. According to elements profile in Fig.3, the distribution of the interfacial reaction products of the SCS-6 SiC/super α2 may be TiC+Ti5Si3, (Ti,Nb)C, (Ti,Nb)3(Si,Al)C2 and (Ti,Nb)3(Al,Si)C from fiber to matrix, which is consistent with the analysis results in Refs.[5, 6].

Fig.1 SEM images of interfacial reaction zone of SCS-6 SiC/super a2 composite: (a) As processed; (b) 700 ℃ for 2 000 h; (c) 800 ℃ for 2 000 h
It is also found that the alloying element Al doesn’t remarkably enter interfacial reaction zone. Compared with it, quite a little amount of Nb appears in the reaction zone. From Refs.[7, 8], the diffusivity of Nb in the ordered phase α2-Ti3Al with D019 structure is around 10-21 m2/s at 800℃, and the diffusivity of Al in this intermetallic compound is 2 to 3 orders of magnitude lower than that of Nb. MARTINEAU et al[9] noted that it was Al atoms which diffuse very slowly, and pile up in front of the matrix-reaction layer interface. So it could be of interest to enhance aluminium concentration to alleviate the reaction rate.

Fig.2 SEM images of interfacial reaction zone of SCS-6 SiC/Ti2AlNb composite: (a) As processed; (b) 700 ℃ for 2 000 h; (c) 800 ℃ for 2 000 h; (d) 900 ℃ for 500 h

Fig.3 Position and results of EDS analysis of interfacial reaction zone: (a), (c) SCS-6 SiC/super a2, 800 ℃ for 2 000 h; (b), (d) SCS-6 SiC/Ti2AlNb, 900 ℃ for 500 h

Fig.4 X-ray diffraction pattern of SCS-6/super a2 after heat treatment at 700 ℃ for 1 000 h
The thermochemical compatibility of the fiber/ matrix interface is of primacy concern in SCS-6 SiC continuous fiber-reinforced Ti-Al intermetallic composites. Interface stability in multi-component system is controlled by a number of chemical, thermo- dynamic and kinetic factors[10]. The SCS-6 SiC fiber was coated by a C-rich layer (about 3 ?m) containing SiC particles which wasn’t stochiometric and crystalline, and a maximum Si concentration appears at 1.5 ?m away from the outermost surface[11]. The interfacial reaction products of SCS-6SiC/Ti2AlNb and SCS-6SiC/ super α2 have been identified as TiC, Ti5Si3 and Ti3Si, and C atoms may carry on long distance diffusion to react with the matrix and form a ternary phase, Ti3AlC[5, 6]. Reaction of C and Si with the Ti-Al intermetallic compound matrix is thermodynamically favored under the present consolidation and heat- treatment conditions as
C+Ti→TiC (1)
3Ti+Si+2C→Ti3SiC2 (2)
3SiC+8Ti→3TiC+Ti5Si3 (3)
3Ti+Al+C→Ti3AlC (4)
For SCS-6 SiC/Ti2AlNb composite the free energy change of the four reactions are -160, -1 326,
-814.9 and -1 924 kJ/mol at 1 000 K, while they are -164.3, -1 337, -842.8 and -1 948 kJ/mol for SCS-6 SiC/super α2 composite[12]. Hence, from the point of view of thermodynamics, the reactions are spontaneous between the SCS-6 SiC fiber and the matrices during the consolidation and the heat treatment of the composites.
3.2 Growth kinetics of interfacial reaction products
From Figs.1 and 2, it can be seen that the reaction zone of the two composites after heat treatment are much thicker than that in the as-processed samples. For example, the reaction layer with thickness of 1.05 and 0.43 ?m was measured, respectively, in the as- processed SCS-6 SiC/super α2 and SCS-6 SiC/ Ti2AlNb, but it becomes 4.04 and 2.58 ?m correspondingly after heat treatment at 800 ℃ for 2 000 h. The measured data of the interfacial reaction layer thickness in the two composites are summarized in Table 1. It can be seen that the temperature is more effective than the time for the reaction layer growth. For example, the reaction layer thickness of 2.87 ?m was measured in SCS-6 SiC/Ti2AlNb heat treated at 900 ℃ for 500 h, but it is only 2.58 ?m at 800 ℃ even for 2 000 h.
Generally, the interfacial reaction is a reaction- controlled process at its very early stage[13]. However, it soon finished and then the reaction was controlled by diffusion when a thin reaction layer formed. The diffusion-controlled growth of the interfacial reaction layer may be satisfied with a parabolic law as[6, 7]
l=kt1/2+b0 (5)
where l is the thickness of the interfacial reaction layer, k is rate constant which is related to the diffusion coefficient of the components and to the thermodyna- mic properties of the interfacial reaction layers, t is time and b0 is the original thickness of the reaction layer in the as-processed samples. Fig.5 shows the relationship between the thickness of the reaction layer and the square root of the reaction time at different temperatures for the two composites.
The data points for each treatment temperature are fitted with a straight line to estimate a generalized reaction rate constant. The fitted equations are as follows.
Table 1 Thickness of interfacial reaction layer under different conditions(μm)
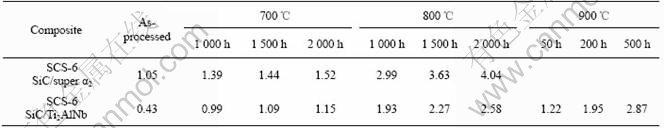

Fig.5 Interfacial reaction kinetic curves of SCS-6 SiC/super α2(a) and SCS-6 SiC/Ti2AlNb(b) at different temperatures
For SCS-6 SiC/super α2 composite,
At 700 ℃
l=1.039×10-2×t1/2+1.051 23 (6)
At 800 ℃
l=6.636×10-2×t1/2+1.018 38 (7)
For SCS-6 SiC/Ti2AlNb composite,
At 700 ℃
l=1.653×10-2×t1/2+0.439 54 (8)
At 800 ℃
l=4.784×10-2×t1/2+0.426 13 (9)
At 900 ℃
l=10.859×10-2×t1/2+0.434 57 (10)
The thickness l is in ?m, and time t is in s for the five equations.
Eqns.(6)-(10) provide quantitative descriptions for the interfacial reactions of the composites and from which the reaction results at a specific time and temperature can be calculated. Furthermore, the fact that the k value increases with increasing reaction temperatures reveals the importance of fabricating these composites at a lower temperature.
In addition the rate constant k in Eqn.(5) follows Arrhenius relation as[9]
![]() (11)
(11)
where k0 is the pre-exponential factor, which is also related to the diffusion coefficient and to the thermodynamic properties, Qk is the growth activation energy, R is the gas constant, and T is the temperature.
Fig.6 shows the Arrhenius plot of the parabolic rate constant of SCS-6 SiC/super α2 and SCS-6 SiC/Ti2AlNb. The values k0 and Qk calculated according to Eqn.(11) are listed in Table 2. Although the value Qk of SCS-6 SiC/Ti2AlNb is 175.709 kJ/mol, far less than that of SCS-6 SiC/super α2, its thickness is thinner compared with that of SCS-6 SiC/super α2. The reason is that probably the matrix Ti2AlNb has higher Nb content. The alloying element Nb remarkably diffuses into the interfacial reaction zone (Fig.3), and displaces some Ti atoms in lattice of interfacial reaction compounds. The results enhance the activation energy for diffusion of the reactive components, and lower the diffusion coefficient. It can be seen from Table 2 that the value of k0 of SCS-6 SiC/Ti2AlNb is only 1.44×10-5 m/s1/2, three orders of magnitude smaller than that of SCS-6 SiC/super α2, so SCS-6 SiC/Ti2AlNb composite has higher interface compatibility and stability. Recently there are some methods to improve the compatibility of fiber/matrix by adjusting the content of Nb and Al. At the premise of not observably weakening creep resistance of Ti-Al alloy at high temperature, Ti2AlNb (O+B2) alloy which contains high concentration of Nb and low concentra tion of Al has not only the favorable interfacial compatibility between the matrix and the fiber, but also the better ductility, rigidity and tensile strength[14].

Fig.6 Arrhenius plot for parabola rate constant of SCS-6 SiC/super α2 and SCS-6 SiC/Ti2AlNb composites
Table 2 Pre-exponential factor and activation energy for layer growth

Dybkov’s reaction diffusion model[15] was used to estimate the mechanism of interfacial reaction in SiCf/Ti and SiC/Ti-15V-3Cr composites[10,16]. If the following three assumptions hold: 1) the reaction layer is large enough and the reaction is a diffusion-controlled process after its fabrication; 2) the growth of interfacial reaction layer is only the growth of TiC layer; 3) the diffusion coefficient of Ti is far less than that of C, the following equations can be drawn:
k=(2DC)1/2 (12)
DC=0.5k2 (13)
where k is rate constant, DC is the diffusion coefficient of C atoms.
From Fig.5 that the growth rate of the reaction zone during the exposure of the SCS-6 SiC/Ti2AlNb and SCS-6 SiC/super α2 composites at high temperature is diffusion controlled. Although the reaction between SCS-6 SiC fiber and the Ti-Al intermetallic compound-matrix produced TiC, Ti5Si3 and Ti3SiC2, It is obvious that the TiC with large equiaxed grains is the main product[5,6]. Others are with small amount. It is known that the diffusivity of C in TiC is around 10-14—10-16 m2/s, and that of Ti in the compound is in the order of 10-21—10-24 m2/s[17, 18]. The diffusion coefficient of C in TiC is at least five orders of magnitude larger than that of Ti in TiC. The diffusion of Ti may be ignored. That is, the growth of interfacial reaction layer is mainly the growth of TiC layer towards the matrix.
Hence, the interfacial reaction in the two SCS-6 SiC/Ti-Al intermetallic compound composites seems consistent roughly with the three assumptions mentioned above and Eqn.(13) can be used to describe the interfacial reaction between SCS-6 SiC fiber and the Ti-Al intermetallic compound matrix.
Substituting k values from Eqns.(6)-(10) into Eqn.(13), the diffusion coefficient of C in TiC for the two composites can be estimated. For SCS-6 SiC/Ti2AlNb, DC is 4.18×10-20 m2/s at 700 ℃, 3.15×10-19 m2/s at 800 ℃, and 1.66×10-18 m2/s at 900 ℃. For SCS-6 SiC/super α2 DC is 1.51×10-20 m2/s at 700 ℃ and 5.85×10-19 m2/s at 800 ℃. These results indicate that the diffusivity of C in reaction layer at 900 ℃ is two orders of magnitude greater than that at 700 ℃ for SCS-6 SiC/Ti2AlNb composite, and the diffusivity of C in reaction layer at 800 ℃ is one order of magnitude greater than that at 700 ℃ for SCS-6 SiC/super α2 composite. The results also indicate that SCS-6 SiC/Ti2AlNb composite has higher interface compatibility and stability at high temperature, especially above 800 ℃.
Not only for SCS-6 SiC/super α2 composite but also for SCS-6 SiC/Ti2AlNb composite, the diffusion coefficient DC is far less than the value in Refs.[17, 18], and the reaction layer is also much thinner than that in TMCs in which the matrix are pure Ti and Ti alloys. It is obvious that, in fact, the estimated diffusion coefficient of C in TiC contains the effects of the microstructure and the interaction between elements. The formation of the TiC during the interfacial reaction follows Eqn.(1). Therefore, Ti in the intermetallics should break the bonding and escape from the compound although its diffusion is not considered. Normally, the bonding between atoms in intermetallics is stronger than that in alloys. From the point of view of thermodynamics, the activity coefficient is a sign of escape ability of element from its crystal lattice. A thermodynamic calculation[19] indicates that the activity coefficient of Ti in the two intermetallics, especially in Ti2AlNb, is much smaller than that in titanium alloys such as Ti-6Al-4V and b-21S. On the other hand, the diffusion of an element can be influenced by other elements in the solid solution [20]. According to the EDS results mentioned above, because of the high contents of Al and Nb in the two intermetallics, the element Al is piled up in front of reaction layer and Nb appears in the reaction zone to substitute some of Ti in the TiC, so written as (Ti,Nb)C, which may decrease the diffusion coefficient of C in the two intermallics matrix composites. Therefore, it is easier to understand that the two IMCs have higher interface compatibility and stability.
4 Conclusions
1) Interfacial reaction takes place in SCS-6 SiC/super α2 and SCS-6 SiC/Ti2AlNb composites. The reaction products are identified as TiC, Ti5Si3, Ti3SiC2 and Ti3AlC and distribute in several layers in the interfacial reaction zone.
2) The growth of the interfacial reaction layers is controlled by diffusion and obeys a parabolic law. The interfacial reaction is more serious in SCS-6 SiC/super α2 than that in SCS-6 SiC/Ti2AlNb.
3)The activation energy for the reaction layer growth is 317.664 kJ/mol for SCS-6 SiC/super α2 and 175.709 kJ/mol for SCS-6 SiC/Ti2AlNb. The value of k0 is 54.4×10-3 m/s1/2 for SCS-6 SiC/super α2 and 1.44×10-5 m/s1/2 for SCS-6 SiC/Ti2AlNb.
4)The diffusion coefficient of C is about 10-18- 10-20 m2/s in TiC in the interfacial reaction zone of SCS-6 SiC/super α2 and SCS-6 SiC/Ti2AlNb compo- sites.
References
[1] LU Pan-quan, ZHOU Sheng-nian. Research progress in continuous fiber reinforced titanium alloy matrix composites [J]. Aviation Production Engineering, 1994(6): 35-37.(in Chinese)
[2] WARD-CLOSE C M, MONOR R, DOORBAR P J. Intermetallic-matrix composites─a review [J]. Intermetallics, 1996, 4: 217-229.(in Chinese)
[3] CAO Yang, LI Guo-jun. The recent development of high-temperature structural intermetallics [J]. Materrials Review, 1994, 4: 14-18.(in Chinese)
[4] YANG Y Q, DUDEK H J, KUMPFERT J. Interfacial reaction and stability of SCS-6SiC/ Ti-25Al-10Nb-3V-1Mo composites [J]. Materials Science and Engineering, 1998, A246: 213-220.(in Chinese)
[5] YANG Y Q, DUDEK H J, KUMPFERT J. Interface stability in SCS-6 SiC/super α2 composites [J]. Scripta Materialia, 1997, 37(4): 503-510.
[6] YANG Y Q, DUDEK H J, KUMPFERT J. TEM investigations of the fiber/matrix interfaces in SCS-6 SiC/Ti-25Al-10Nb-3V-1Mo composites [J]. Composites Part A, 1998, 29A: 1235-1241.
[7] BREUER J, WILGER T, FRIESEL M, et al. Interstitial and substitutional diffusion of metallic solutes in Ti3Al [J]. Intermetallics, 1999, 7: 381-388.
[8] J?rg Rüsing, Christian Herzig. Titanium self-diffusion and chemical diffusion in Ti3Al [J]. Scripta Metall Materialia, 1995, 33(4): 561-566.
[9] MARTINEAU P, PAILLER R, LAHAVE M, et al. SiC filament/titanium matrix composites regarded as model composites(part 2)─fibre/matrix chemical interactions at high temperatures [J]. Journal of Materials Science, 1984, 19: 2749-2770.
[10] XUN Y W, TAN M J, ZHOU J T. Processing and interface stability of SiC fiber reinforced Ti-15V-3Cr matrix composites [J]. Journal of Materials Processing Technology, 2000, 102: 215-220.
[11] NING X J, PIROUZ P. The microstructure of SCS-6 SiC fiber [J]. J Mater Res, 1991, 6(10): 2234-2248.
[12] ZHU Yan. Study on the Interfacial Reactions of SiC Fiber Reinforced Ti-Matrix Composites[D]. Xi’an: Northwestern Polytechnical University, 2003.(in Chinese)
[13] GUO Z X, DERBY B. Interfaces in Ti3Al composites reinforced with sigma SiC fibers [J]. Scripta Metall Mater, 1994, 30: 89-94.
[14] SI Yu-feng, MENG Li-hua, WANG Zhao-wen, et al. Research progress in Ti3Al base intermetallic compound [J]. Special Casting & Nonferrous Alloys, 2003(4): 33-35.(in Chinese)
[15] DYBKOV V I. Reaction diffusion in heterogeneous binary systems(part 1)─growth of the chemical conpound layers at the interface between two elementary substances: one compound layer [J]. Journal of Materials Science, 1986, 21: 3078-3084.
[16] ZHANG Guo-xing, KANG Qiang, SHI Nan-lin, et al. Kinetics and mechanism of interfacial reaction in a SiCf/Ti composite [J]. J Mater Sci Technol, 2003, 19(5): 407-410.
[17] FROST H J. Deformation Mechanism Maps [M]. Oxford: Pergamon Press, 1982. 80.
[18] BACKHAUS-RICOULT M. Metal-Ceramic Interfaces [M]. Oxford: Pergamon Press, 1990. 79.
[19] ZHU Yan, YANG Yan-qing, SUN Jun. Calculation of activity coefficients for components in ternary Ti alloys and intermetallics as matrix of composites [J]. Trans Nonferrous Met Soc China, 2004, 14: 875-879.
[20] QI Zheng-feng. Diffusion and Phase Transitions in Solids [M]. Beijing: China Machine Industry Press, 1998. 123.(in Chinese)
Foundation item: Project(50371069) suppported by the National Natural Science Foundation of China; Project(20030699013) suported by the State Educational Ministry Doctoral Foundation; Project(04G53044) supported by the Foundation of Aviation Science; Project(ZX200301014) supported by the Materials Engineering Center Foundation of Jiangxi Province, China
Correspondence author: L? Xiang-hong; Tel: +86-29-88486091; E-mail: lxhong71@mail.nwpu.edu.cn
(Edited by LONG Huai-zhong)

