高温自蔓延过程中的挤压工艺对合成Ni3Al-B-Cr合金的组织
来源期刊:中国有色金属学报(英文版)2012年第3期
论文作者:盛立远 奚廷斐 赖琛 郭建亭 郑玉峰
文章页码:489 - 495
关键词:Ni3Al 金属间化合物;高温自蔓延合成;挤压;微观组织;力学性能
Key words:Ni3Al intermetallic compound; self-propagation high-temperature synthesis; extrusion; microstructure; mechanical properties
摘 要:
采用高温自蔓延及挤压工艺制备Ni3Al-0.5B-5Cr合金,研究挤压工艺对合成合金的微观组织及力学性能的影响。结果表明:合成后的挤压工艺可使合成合金进一步致密并能有效地细化其组织。X射线衍射及透射电镜观察发现除了Ni3Al基体外,合金中还含有Al2O3、Ni3B 及 Cr3Ni2析出相。与无挤压合成的合金有所不同,合金在高温自蔓延合成及挤压过程中经历了大变形和再结晶过程,其促进了组织的细化并降低了晶粒的取向差。此外,合成后的挤压工艺促使Al2O3颗粒重新分布且减少了γ-Ni相。与无挤压合成的合金相比,高温自蔓延合成及挤压工艺制备的合金具有更好的室温力学性能。
Abstract:
The well-densified Ni3Al-0.5B-5Cr alloy was fabricated by self-propagation high-temperature synthesis and extrusion technique. Microstructure examination shows that the synthesized alloy has fine microstructure and contains Ni3Al, Al2O3, Ni3B and Cr3Ni2 phases. Moreover, the self-propagation high-temperature synthesis and extrusion lead to great deformation and recrystallization in the alloy, which helps to refine the microstructure and weaken the misorientation. In addition, the subsequent extrusion procedure redistributes the Al2O3 particles and eliminates the γ-Ni phase. Compared with the alloy synthesized without extrusion, the Ni3Al-0.5B-5Cr alloy fabricated by self-propagation high-temperature synthesis and extrusion has better room temperature mechanical properties, which should be ascribed to the microstructure evolution.

![]()

Trans. Nonferrous Met. Soc. China 22(2012) 489-495
SHENG Li-yuan1, 2, XI Ting-fei1, LAI Chen1, GUO Jian-ting3, ZHENG Yu-feng2
1. PKU-HKUST Shenzhen-Hong Kong Institution, Shenzhen 518057, China;
2. College of Engineering, Peking University, Beijing 100871, China;
3. Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China
Received 9 September 2011; accepted 16 January 2012
Abstract: The well-densified Ni3Al-0.5B-5Cr alloy was fabricated by self-propagation high-temperature synthesis and extrusion technique. Microstructure examination shows that the synthesized alloy has fine microstructure and contains Ni3Al, Al2O3, Ni3B and Cr3Ni2 phases. Moreover, the self-propagation high-temperature synthesis and extrusion lead to great deformation and recrystallization in the alloy, which helps to refine the microstructure and weaken the misorientation. In addition, the subsequent extrusion procedure redistributes the Al2O3 particles and eliminates the γ-Ni phase. Compared with the alloy synthesized without extrusion, the Ni3Al-0.5B-5Cr alloy fabricated by self-propagation high-temperature synthesis and extrusion has better room temperature mechanical properties, which should be ascribed to the microstructure evolution.
Key words: Ni3Al intermetallic compound; self-propagation high-temperature synthesis; extrusion; microstructure; mechanical properties
1 Introduction
Nickel aluminide intermetallic alloys (NiAl and Ni3Al) have received considerable attention for high-temperature structural and coating applications, e.g., as heat shields for combustion chambers and as first-row vanes in industrial gas turbines [1,2]. In addition, numerous alloys based upon Ni3Al have been developed with broad utilizations ranging from furnace rolls and radiant burner tubes for steel production to heat treating fixtures, forging dies, and corrosion-resistant parts for chemical industries [3,4]. This is because strong bonding between aluminum and nickel, which persists at elevated temperatures, yields excellent properties competitive with those of superalloys and ceramics, such as high melting point, low densities, high strength, as well as good corrosion and oxidation resistance [1-4]. In spite of these attractive properties, however, low ductility, brittle fracture, and processing problems were the major disadvantages of nickel aluminides [3-5]. The brittleness of Ni3Al stems from an environmental effect caused by hydrogen generated through the reduction of moisture in air by aluminum in the aluminides [4,6]. A major breakthrough to resolve this issue is the discovery of the dramatic effects of boron addition on ductility improvement for Ni3Al at ambient and high temperatures. LIU et al [7] found that 50% tensile ductility can be achieved in the Ni3Al alloy with the addition of small amount of boron up to 0.4%. Interestingly, as the Al content of B-doped Ni3Al is decreased to below 25% (mole fraction), the ductility increases significantly and the fracture mode changes from brittle intergranular to ductile transgranular. However, the ductility drops drastically to about 5% when the Al content is increased to 25% (mole fraction) or higher.
MORSI [1] reviewed a number of novel processes applied to the reaction synthesis of Ni-Al intermetallics. Among them, combustion synthesis with the advantages of time and energy savings has been recognized as a promising alternative to the conventional methods of producing advanced materials, including carbides,borides, nitrides, hydrides, and intermetallics, etc [8-11]. Combustion synthesis of the Ni3Al intermetallic can be conducted in either of two modes, the self-propagating high-temperature synthesis (SHS) [12,13] and the thermal explosion [14,15]. By using combustion synthesis in the SHS mode with powder compacts, LEBRAT and VARMA [13] found that higher green density and preheating temperature led to fully reacted product with a well-developed microstructure.
Though the synthesis of Ni3Al has been extensively studied, the porosity is still its main problem. The hot extrusion could solve the trouble, but it resulted in the crystal coarsening. The recent studies [12,13] reveal that the combustion synthesis and hot extrusion can get well-densified materials. Therefore, in the present work, the Ni3Al-0.5B-5Cr (Ni3Al-B-Cr for short) alloy was fabricated by SHS with and without extrusion, and their microstructure and mechanical properties were investigated.
2 Experimental
Two kinds of powders, including powders of nickel (with an average particle size of 0.98 μm) and aluminum (1.45 μm) were used as the initial materials. Besides the main constituents, boron powders (3 μm) and chromium powders (0.92 μm) were used as the additive. Powder mixtures with nominal composition (n(Ni):n(Al):n(Cr): n(B)=71:23.5:5:0.5) were dry mixed in a ball milling for 10 h. The mixed powders were compacted into a cylinder with dimensions of d40 mm×40 mm, and then the powder compact was put into the SHS with extrusion synthesis system, as shown in Fig. 1(a). Firstly, the induction coil heated the reaction puncheon rapidly to 760 K to start the reaction synthesis. A thermocouple in the SHS synthesis system was used to measure the temperature of the powder mixtures. When the temperature increased dramatically, it signaled the start of reaction synthesis. Then, two seconds later, a pressure of 400 MPa would load on the reaction puncheon in order to extrude the synthesized Ni3Al out of the reaction floor through a hole with diameter of 6 mm. The as-fabricated sample is shown in Fig. 1(b). In order to investigate the effect of the extrusion, a sample without extrusion was fabricated in the present work.
The specimens for microstructure observation and compression test were cut from the center of the extruded part in sample with hot extrusion and the centre of the sample without extrusion. The resultant phases in the different alloys were characterized by X-ray diffraction (XRD) with a Cu Kα radiation at 40 kV and 40 mA. Microstructural characterizations of all samples were carried out on an OLYMPUS GX41 optical microscope (OM). Samples for OM observations were prepared by conventional methods of mechanical polishing and chemical etching with an acidic mixture (V(CH3COOH):V(HNO3):V(HCl)=8:4:1). The foils for transmission electron microscopy (TEM) observation were prepared by the conventional twin jet polishing technique using an electrolyte of 10% perchloric acid in methnol at –20 °C after mechanically polishing to 50 μm and cutting into disc with a diameter of 3.0 mm. The TEM observation was performed on a JEM-2010 transmission electron microscope operated at 200 kV.
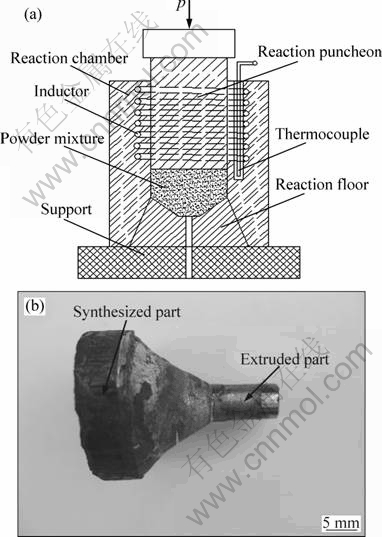
Fig. 1 Schematic diagram of self-propagation high-temperature synthesis and extrusion synthesis system (a), and appearance of synthesized sample by SHS with extrusion (b)
Microhardness measurement was carried out on a Vickers microhardness tester (MHV-2000) using a load of 1.47 N and a dwell time of 15 s. Seven measurements were performed to evaluate an average value. The compressive specimens with dimensions of 4 mm×4 mm×6 mm were cut from the samples and all surfaces were mechanically ground with 600-grit SiC abrasive prior to compression test. The compression was conducted on a Gleeble-1500 test machine at room temperature (RT), with an initial strain rate of 1×10-3 s-1.
3 Results and discussion
3.1 Microstructure
X-ray diffraction patterns reveal that the elemental powders were transformed to the Ni3Al phase after SHS processing, as shown in Fig. 2. From the XRD pattern of the sample without extrusion, it can be seen that the B and Cr additions have not led to any changes. There is no any other phase except the Ni3Al. However, the extrusion subsequent following the SHS exerts great influence on the microstructure. The extrusion leads to the great increase of [111] peak, which indicates that the sample has a strong tendency along [111]. In addition, it also shows that there are obvious peaks of Al2O3, Cr3Ni2 and Ni3B phases, and moreover the peak of Al2O3 is strong. Such a difference should be mainly attributed to the great deformation and the short ageing time caused by the residual heat. Based on the previous research [16], the complex effect of ageing and great deformation can promote the precipitation.

Fig. 2 X-ray diffraction patterns of Ni3Al-B-Cr alloy synthesized by SHS with and without extrusion
The microstructures of all samples prepared by SHS with and without extrusion are shown in Fig. 3. It is clear that the sample without hot extrusion still has big cavity, and has coarse and fine grains simultaneously with size from 2 to 40 μm, as shown in Fig. 3(a). Twinned Ni3Al crystals are easy to be found in the sample without extrusion. The Al2O3 dispersoids segregate along the Ni3Al grain boundary. The sample with extrusion exhibits fine and homogeneous grain size and less porosity, as shown in Fig. 3(b). Compared with the sample without extrusion, it can be found that the amount of Al2O3 in the sample with extrusion increases a little, and these Al2O3 dispersoids prefer to aggregate and distribute along the grain boundary. The origin of Al2O3 dispersoids can be explained by either the fracture of the oxide layer covering the original powder particles or powder oxidation during the milling process. The formation of Al2O3 dispersoids and their effect on the formation of a microstructure with coarse and fine grains have been explained elsewhere [12,13].
The results of TEM observations on the sample without extrusion are presented in Fig. 4. It is clear that the sample is mainly composed of Ni3Al phase, but it still consists of other phases, as shown in Fig. 4(a). The γ-Ni phase with size of several micrometers exists in the sample, which may be ascribed to the elements segregation. Additionally, the α-Cr particles with hundreds of nanometers are observed in the Ni3Al matrix, as shown in Fig. 4(b). Based on the recent study [17], the amount of Cr addition in the present work is less than its solubility in Ni3Al. Therefore, the precipitation of α-Cr particles should be attributed to the relatively low cooling rate, according to the previous research [18]. In addition, the Al2O3 particle is observed along Ni3Al grain boundary, as shown in Fig. 4(c). The inset selected area electron diffraction (SAED) pattern reveals that it is θ-Al2O3. The existence of such a metastable phase may be explained by relatively rapid synthesis process, which handicaps its transformation. The TEM observations also certify the twinned Ni3Al crystal, as shown in Fig. 4(d). Previous research has exhibited that the annealing treatment on high-deformed Ni3Al polycrystals can lead to the formation of twinned Ni3Al [18]. In the present investigation, the pre-compaction can generate great stress in the sample, which may contribute to the formation of the twinned Ni3Al.
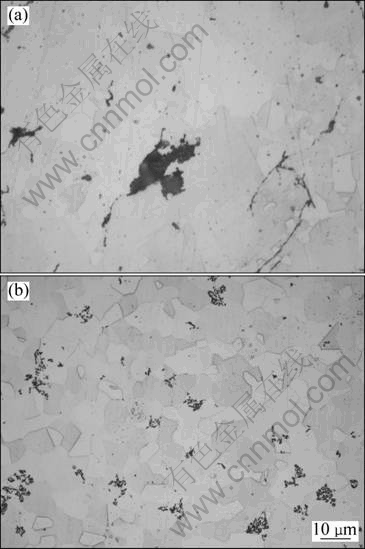
Fig. 3 Optical micrographs of Ni3Al-B-Cr alloy synthesized by SHS: (a) Without extrusion, (b) With extrusion
TEM observations on the sample with hot extrusion reveal that ultrafine Ni3Al grains with hundreds nanometers exist in the extruded part, as shown in Fig. 5(a). Along the Ni3Al grain boundary, the Cr3Ni2 precipitate is observed, as shown in Fig. 5(b). According to the recent research [17], such a phase would precipitate in a short ageing time. So, it can be deduced that the great stress and relatively low cooling rate at moderate temperature promote the formation of Cr3Ni2 phase. Moreover the small Ni3B particle is observed in the extruded part, as shown in Fig. 5(c). Further observations along the Ni3Al grain boundary reveal that the Al2O3 particles segregate, as shown in Fig. 5(d). The evolution of the sample with hot extrusion compared with the sample without hot extrusion should mainly attribute to the great deformation, which promotes the microstructure refinement and element diffusion.
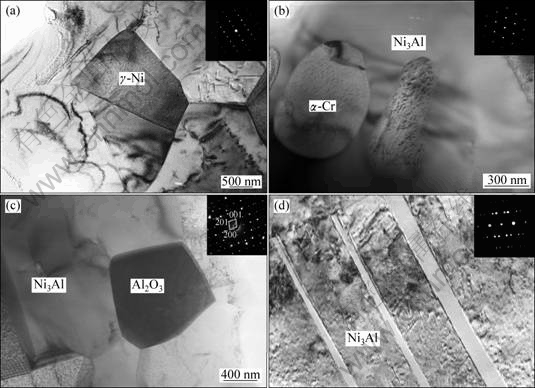
Fig. 4 TEM bright-field images of Ni3Al-B-Cr alloy synthesized by SHS without extrusion: (a) Morphology of γ-Ni in matrix; (b) Precipitation of α-Cr-rich particle in Ni3Al grain; (c) Formation of θ-Al2O3 along grain boundary; (d) Morphology of twinned Ni3Al (Insets show SAED patterns of corresponding phases)

Fig. 5 TEM bright-field images of Ni3Al-B-Cr alloy synthesized by SHS with extrusion: (a) Morphology of ultrafine Ni3Al in extruded part; (b) Precipitation of Cr3Ni2 phase along Ni3Al grain boundary; (c) Formation of Ni3B precipitate in fine Ni3Al region; (d) Segregation of fine Al2O3 particles along Ni3Al grain boundary (Insets show SAED patterns of corresponding phases)
Further observations on the Al2O3 in the extruded part reveal that the Al2O3 particles have two different kinds of structures. The morphology of the Al2O3 particles and their selected area electron diffraction (SAED) patterns are exhibited in Fig. 6. One has the hexagonal crystal structure with ![]() space group and the cell parameters: a=b=0.4758 nm, c=1.299 nm, as shown in Figs. 6(a)-(c). This Al2O3 can be determined as α-Al2O3, which is the most stable one in all Al2O3. The other one has the face-centered crystal structure with
space group and the cell parameters: a=b=0.4758 nm, c=1.299 nm, as shown in Figs. 6(a)-(c). This Al2O3 can be determined as α-Al2O3, which is the most stable one in all Al2O3. The other one has the face-centered crystal structure with ![]() space group and cell parameters: a=b=c=0.7948 nm, as shown in Figs. 6(d)-(e). This Al2O3 is determined as the γ-Al2O3, which is a metastable phase and will transform into α-Al2O3 when heat-treated above 1200 K. The existence of the two kinds of Al2O3 should be attributed to the synthesis process. The high pressure and rapid heating process lead to the formation of Al2O3 with different structures, while the short time at high temperature keeps the structure of the Al2O3.
space group and cell parameters: a=b=c=0.7948 nm, as shown in Figs. 6(d)-(e). This Al2O3 is determined as the γ-Al2O3, which is a metastable phase and will transform into α-Al2O3 when heat-treated above 1200 K. The existence of the two kinds of Al2O3 should be attributed to the synthesis process. The high pressure and rapid heating process lead to the formation of Al2O3 with different structures, while the short time at high temperature keeps the structure of the Al2O3.
The difference between these two kinds of samples is the extrusion procedure after the self-propagation high-temperature synthesis. The morphology of the extruded part of the sample with the extrusion is shown in Fig. 7(a). It is obvious that the Ni3Al matrix deforms drastically. The Ni3Al grains are elongated along the extrusion direction. Compared with the sample without hot extrusion, the size of the Ni3Al grains in the extruded part is refined significantly. From the results it can be concluded that the Ni3Al grains are fragmentized and then recrystallized in the following process. But due to the short time at high temperature, the recrystallization is incomplete. So, in the extruded part there are massive dislocations in many Ni3Al grains, which result in many substructures. As shown in Fig. 7(b), the dislocations in the big Ni3Al grain have regular array, which indicates the deformation crystal plane. While in the small Ni3Al grain, the dislocations interact and accumulate in the grain boundary, as shown in Fig. 7(c).
3.2 Mechanical properties
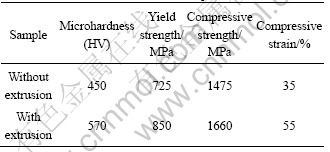
The microhardness and compressive properties at room temperature (RT) of the samples with and without extrusion are shown in Table 1. It is clear that the mechanical properties of the sample with extrusion are better than those of the sample without extrusion. Especially the compressive strain increases by more than 50%, compared with the sample without extrusion. The hardness and strength of the sample with extrusion increase by 15%-25%. The previous studies [19,20] on the Ni3Al find that the grain size has great effect on the yield strength and its ductility. With the decrease of grain size, the yield strength and ductility at room temperature of the Ni3Al increase obviously. In the present investigation, the extrusion following the SHS refines the Ni3Al. It can be believed that it would improve the yield strength of the Ni3Al further. Additionally, the TEM observation on the extruded part shows a lot of dislocations generated from the grain boundary. According to the researches [21,22], these dislocations act as sources for emitting matrix dislocations, which would improve the ductility and fracture toughness of the materials.
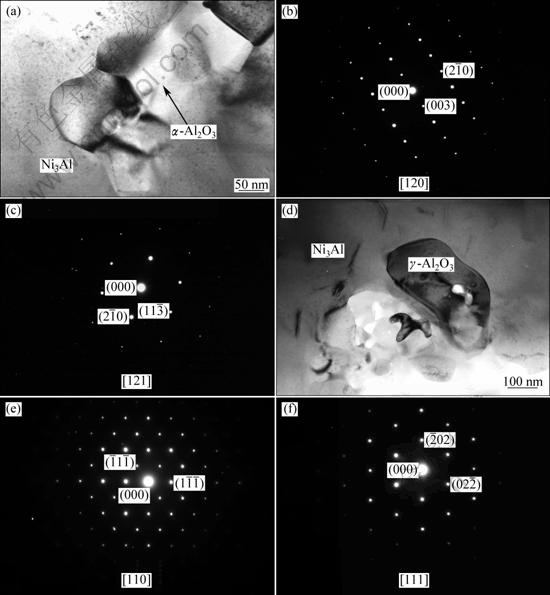
Fig. 6 TEM images of α-Al2O3 particle (a) and SAED patterns along [120] (b) and [121] (c), TEM micrograph of γ-Al2O3 particle (d) and SAED patterns along [110] (e) and [111] (f)
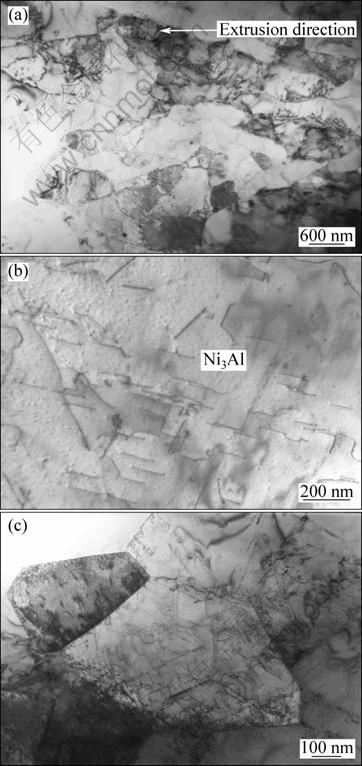
Fig. 7 TEM images of extruded part: (a) Deformed grains along extrusion direction; (b) Morphology of dislocation array in Ni3Al matrix; (c) Morphology of dislocations in fine Ni3Al grain
Table 1 Mechanical properties of samples prepared by SHS with and without extrusion at room temperature
Previous research [23] on the Ni3Al revealed that great deformation could result in microcrystalline with high density of defects (dislocations, twins, slip bands) by fragmentation, mechanical twinning of the initial crystals. In the present investigation, the extrusion process after the SHS leads to great deformation in the Ni3Al-B-Cr alloy. Based on the above observation, it can be concluded that the great pressure extrudes the synthesized alloy out. During the extrusion procedure, a funnel-shaped flowing zone is formed and the funnel-shaped flowing zone results in the break of the bulk Ni3Al grain into microcrystalline. Moreover, the microcrystalline is twisted at the end of the funnel-shaped flowing zone, which makes the microcrystalline have weak misorientation. At last, the extruded Ni3Al with homogeneous and fine microstructure and less porosity is obtained, which improves its mechanical properties significantly. In addition, the dispensed Al2O3 oxides along the grain boundary contribute to the fragmentation of massive grains. In return, the fragmentation makes the Al2O3 oxides distribute more uniformly, which is beneficial to the strength and ductility. Therefore, it can be seen that the sample with extrusion has better mechanical properties.
4 Conclusions
1) The self-propagation high-temperature synthesis and subsequent extrusion densify the Ni3Al-0.5B-5Cr alloy and refine the microstructure obviously.
2) Besides the Ni3Al, the alloy synthesized by self-propagation high-temperature synthesis still contains θ-Al2O3, γ-Ni, α-Cr and NiAl particles. While in the alloy synthesized by self-propagation high-temperature synthesis and extrusion, α-Al2O3, γ-Al2O3, Cr3Ni2 and Ni3B particles become the main precipitates.
3) The alloy synthesized by self-propagation high-temperature synthesis and extrusion experiences great deformation and recrystallization, which contributes to the microstructure refinement and weakens the misorientation.
4) Compared with the synthesized alloy without hot extrusion, the self-propagation high-temperature synthesis and extrusion improve the Ni3Al-0.5B-5Cr alloy obviously, especially the ductility, which increases by almost 60%.
References
[1] MORSI K. Review: Reaction synthesis processing of Ni-Al intermetallic materials [J]. Materials Science and Engineering A, 2001, 299(1-2): 1-15.
[2] SCHEPPE F, SAHM P R, HERMANN W, PAUL U, PREUHS J. Nickel aluminides: A step toward industrial application [J]. Materials Science and Engineering A, 2002, 329-331: 596-601.
[3] STOLOFF N S, LIU C T, DEEVI S C. Emerging applications of intermetallics [J]. Intermetallics, 2000, 8(9-11): 1313-1320.
[4] SIKKA V K, DEEVI S C, VISWANATHAN S, SWINDEMAN R W, SANTELLA M L. Advances in processing of Ni3Al-based intermetallics and applications [J]. Intermetallics, 2000, 8(9-11): 1329-1337.
[5] DEEVI S C, SIKKA V K. Nickel and iron aluminides: an overview on properties, processing, and applications [J]. Intermetallics, 1996, 4(5): 357-375.
[6] GEORGE E P, LIU C T, POPE D P. Intrinsic ductility and environmental embrittlement of binary Ni3Al [J]. Scripta Metall Mater, 1993, 28(7): 857-862.
[7] LIU C T, WHITE C L, HORTON J A. Effect of boron on grain-boundaries in Ni3Al [J]. Acta Metall, 1985, 33(2): 213-229.
[8] MUNIR Z A, ANSELMI-TAMBURINI U. Self-propagating exothermic reactions: The synthesis of high-temperature materials by combustion [J]. Materials Science Reports, 1989, 3(6): 277-365.
[9] MERZHANOV A G. History and recent developments in SHS [J]. Ceramics International, 1995, 21(5): 371-379.
[10] MOORE J J, FENG H J. Combustion synthesis of advanced materials: Part I. Reaction parameters [J]. Progress in Materials Science, 1995, 39(4-5): 243-273.
[11] MOSSINO P. Some aspects in self-propagating high-temperature synthesis [J]. Ceramics International, 2004, 30(3): 311-332.
[12] GUO J T, SHENG L Y, XIE Y, ZHANG Z X, OVCHARENKO V E, YE H Q. Microstructure and mechanical properties of Ni3Al and Ni3Al–1B alloys fabricated by SHS/HE [J]. Intermetallics, 2011, 19(2): 137-142.
[13] LEBRAT J P, VARMA A. Self-propagating high-temperature synthesis of Ni3Al [J]. Combust Sci Technol, 1992, 88(3-4): 211-221.
[14] HIBINO A, MATSUOKA S, KIUCHI M. Synthesis and sintering of Ni3Al intermetallic compound by combustion synthesis process [J]. J Mater Process Technol, 2001, 112(1-3): 127-135.
[15] SHENG L Y, ZHANG W, GUO J T, WANG Z S, OVCHARENKO V E, ZHOU L Z, YE H Q. Microstructure and mechanical properties of Ni3Al fabricated by thermal explosion and hot extrusion [J]. Intermetallics, 2009, 17(7): 572-577.
[16] DUTTA B, PALMIERE E J, SELLARS C M. Modelling the kinetics of strain induced precipitation in Nb microalloyed steels [J]. Acta Mater, 2001, 49: 785-794.
[17] SHENG L Y, XIE Y, XI T F, GUO J T, ZHENG Y F, YE H Q. Microstructure characteristics and compressive properties of NiAl-based multiphase alloy during heat treatments [J]. Materials Science and Engineering A, 2011, 518: 8324-8331.
[18] SHENG L Y, WANG L J, XI T F, ZHENG Y F, YE H Q. Microstructure, precipitates and compressive properties of various holmium doped NiAl/Cr(Mo, Hf) eutectic alloys [J]. Mater Design, 2011, 32(10): 4810-4817.
[19] SCHULSON E M, WEIHS T P, VIENS D V, BAKER I. The effect of grain size on the yield strength of Ni3Al [J]. Acta Metall, 1985, 33(9): 1587-1591.
[20] TAKEYAMA M, LIU C T. Effects of grain size and test temperature on ductility and fracture behavior of A B-doped Ni3Al alloy [J]. Acta Metall, 1988, 36(5): 1241-1249.
[21] SHENG L Y, ZHANG W, GUO J T, ZHOU L Z, YE H Q. Microstructure evolution and mechanical properties improvement of NiAl-Cr(Mo)-Hf eutectic alloy during suction casting and subsequent HIP treatment [J]. Intermetallics, 2009, 17(12): 1115-1119.
[22] WUNDERLICH W, KREMSER T H, FROMMEYER G. Mobile dislocations at the α2/γ phase boundaries in intermetallic TiAl/Ti3Al-alloys [J]. Acta Metall Mater, 1993, 41(6): 1791-1799.
[23] KORZNIKOV A V, TRAM G, DIMITROV O, KORZNIKOVA G F, IDRISOVA S R, PAKIELA Z. The mechanism of nanocrystalline structure formation in Ni3Al during severe plastic deformation [J]. Acta Mater, 2001, 49(4): 663-671.
盛立远1, 2,奚廷斐1,赖 琛1,郭建亭3,郑玉峰2
1. 北京大学-香港科技大学 深圳研修院,深圳 518057;
2. 北京大学 工学院,北京 100871;
3. 中国科学院 金属研究所,沈阳 110016
摘 要:采用高温自蔓延及挤压工艺制备Ni3Al-0.5B-5Cr合金,研究挤压工艺对合成合金的微观组织及力学性能的影响。结果表明:合成后的挤压工艺可使合成合金进一步致密并能有效地细化其组织。X射线衍射及透射电镜观察发现除了Ni3Al基体外,合金中还含有Al2O3、Ni3B 及 Cr3Ni2析出相。与无挤压合成的合金有所不同,合金在高温自蔓延合成及挤压过程中经历了大变形和再结晶过程,其促进了组织的细化并降低了晶粒的取向差。此外,合成后的挤压工艺促使Al2O3颗粒重新分布且减少了γ-Ni相。与无挤压合成的合金相比,高温自蔓延合成及挤压工艺制备的合金具有更好的室温力学性能。
关键词:Ni3Al 金属间化合物;高温自蔓延合成;挤压;微观组织;力学性能
(Edited by LI Xiang-qun)
Foundation item: Project (2012CB933600) supported by the National Basic Research Program of China; Project (2011AA030104) supported by the National High-tech Research and Development Program of China; Project (JC200903170498A) supported by the Science and Technology Research Foundation of Shenzhen Bureau of Science and Technology & Information, China
Corresponding author: SHENG Li-yuan; Tel: +86-18665806226; Fax: +86-755-26984814; Email: lysheng@yeah.net
DOI: 10.1016/S1003-6326(11)61203-X

