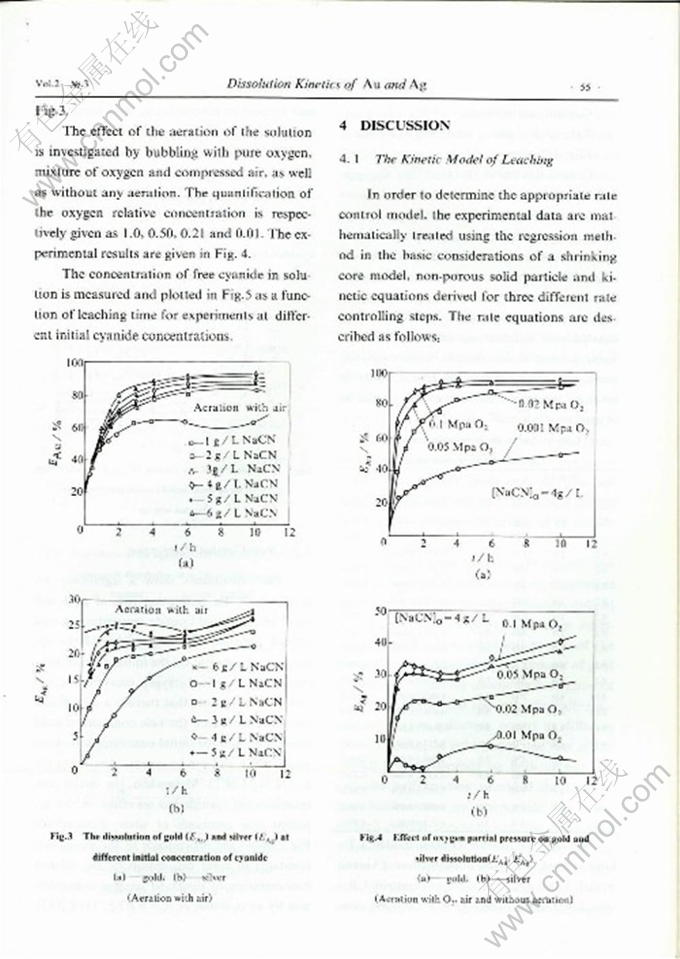
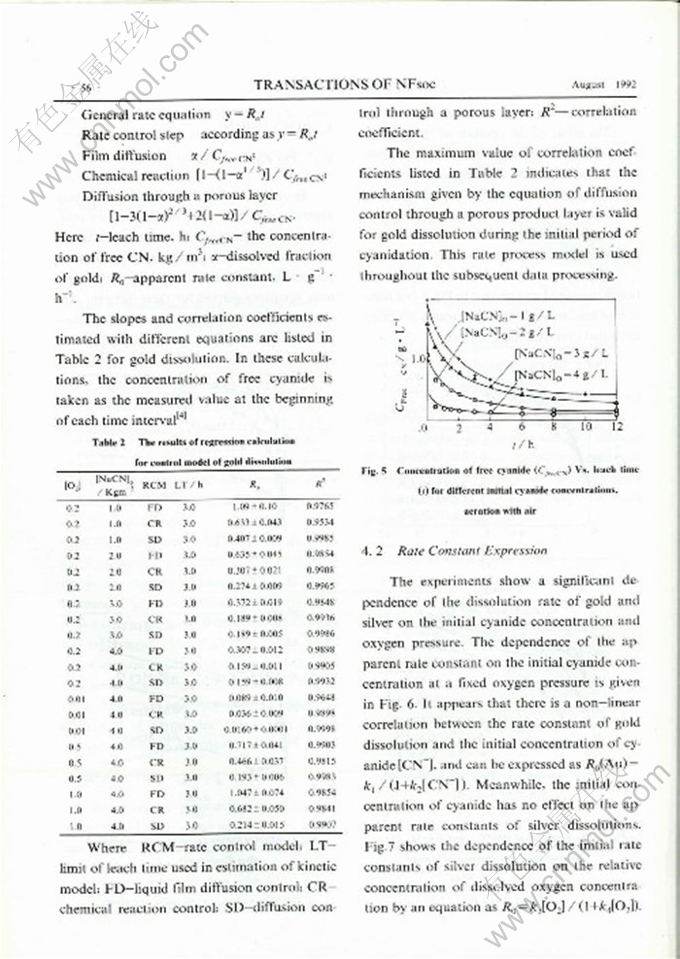
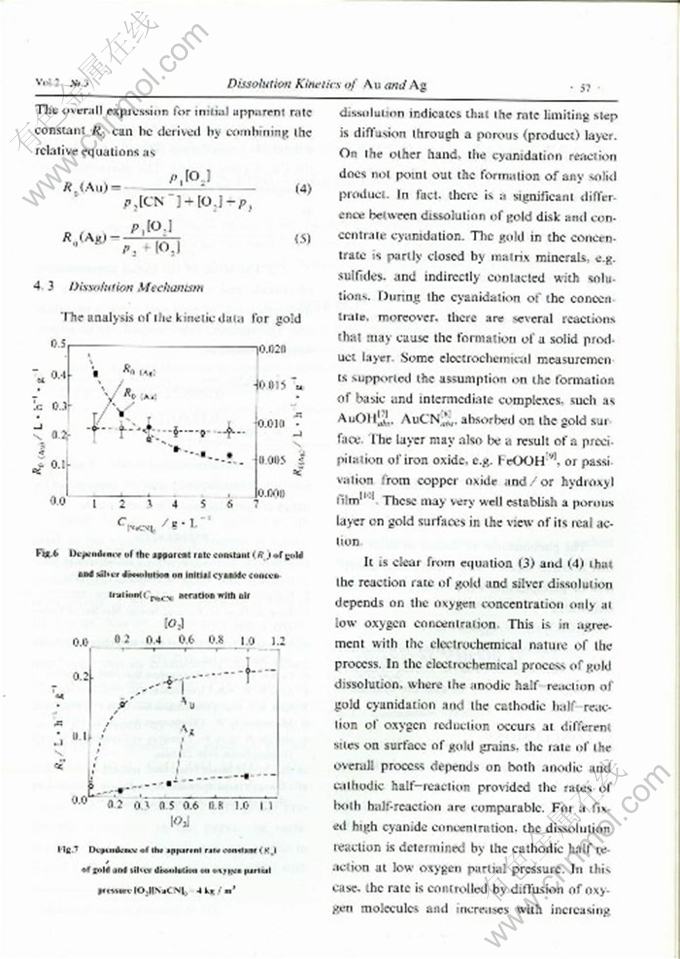
DISSOLUTION KINETICS OF GOLD AND SILVER IN CYANIDATION
来源期刊:中国有色金属学报(英文版)1992年第3期
论文作者:Fang Zhaoheng Mamoun Muhammed
文章页码:53 - 58
Key words:dissolution kinetics; Cu-Au sulfide concentrate ; cyanidation ; electrochemical mechanism
Abstract: The dissolution kinetics of gold and silver cyanidation of Cu-Au sulFide concentrate has been investigated at ambient temperature in consideration of effects of various parameters, such as particle size of ores, hydrodynamics of the process and initial cyanide concentration as well as oxygen partial pressure. The experimental data are mathematically treated with an approach based on the shrinking core model. A phenomenological expression describing the rate and rate constants for cyanidation of the concentrate is developed from the treatment. The dissolution of gold and silver is explained by an electrochemical mechanism in which the rate determining step is the diffusion of cyanide and dissolved molecular oxygen through a porous layer formed during the minerals dissolutions