文章编号:1004-0609(2011)11-2958-06
NaOH-NaNO3熔盐法分解铁尾矿的动力学
于洪浩1, 2, 贺 燕1, 薛向欣2, 王余莲3
(1. 沈阳理工大学 材料科学与工程学院,沈阳 110159;2. 东北大学 材料与冶金学院,沈阳 110004;
3. 东北大学 资源与土木工程学院,沈阳 110004)
摘 要:对铁尾矿在NaOH-NaNO3熔盐体系中的分解动力学进行研究,考察了反应温度、反应时间及碱渣比对铁尾矿中SiO2浸出率的影响。结果表明:铁尾矿在NaOH-NaNO3熔盐体系中的分解符合未反应收缩核模型,受固体产物层内扩散控制,提高反应温度、延长反应时间或增加碱渣比均能提高铁尾矿的分解率;在一定的操作条件下,根据Arrherius方程得到铁尾矿在NaOH-NaNO3亚熔盐体系中的反应活化能为17.43 kJ/mol。
关键词:铁尾矿;熔盐法;NaOH-NaNO3;动力学
中图分类号:TB 321 文献标志码:A
Decomposition kinetics of iron ore tailings by NaOH-NaNO3 molten salt method
YU Hong-hao1, 2, HE Yan1, XUE Xiang-xin2, WANG Yu-lian3
(1. School of Material Science and Engineering, Shenyang Ligong University, Shenyang 110159, China;
2. School of Material and Metallurgy, Northeastern University, Shenyang 110004, China;
3. School of Resource and Civil Engineering, Northeastern University, Shenyang 110004, China)
Abstract: The decomposing kinetics of iron ore tailings in NaOH-NaNO3 molten salt was investigated. The effects of reaction temperature, reaction time and NaOH-to-SiO2 ratio on leaching rate of SiO2 in iron ore tailing were also studied. The results show that the decomposing process of iron ore tailings in NaOH-NaNO3 is well interpreted with the unreacted shrinking core model under diffusion reaction control. The decomposition rate of iron ore tailings increases with increasing the reaction temperature, reaction time and NaOH-to-SiO2 ratio. Under the specific operating conditions, the activation energy for the decomposition of iron ore tailings is calculated according to Arrhenius expression. The apparent activation energy is 17.43 kJ/mol as calculated from the experimental data.
Key words: iron ore tailings; molten salt method; NaOH-NaNO3; kinetics
随着近年来钢铁工业的迅速发展,固体废料中铁尾矿所占的比例也越来越大,几乎占全部尾矿堆存总量的1/3[1-7]。这些尾矿废料多以自然堆积法存储于尾矿库中,不仅要侵占土地,耗费建库资金,而且由于尾矿颗粒一般较细,容易被风扬起,经常造成周围地区的局部沙尘暴,遭遇洪水时,又常由于库坝决口而造成泥石流灾害[8-9]。同时,我国又是一个资源相对短缺,然而又不得不依靠消耗大量原材料进行经济建设的国家。很明显,若使用铁尾矿作为二次资源开发进行利用[10-12],无疑将对我国的可持续发展产生重大而深远的影响。
熔盐法是利用在常压下流动的高浓介质中的拟均相反应分解矿石,可极大地强化反应和质量、热量传递,在相对较低的温度下获得较高的矿石分解率。与传统碱溶-浸出法相比,由于熔盐体系能够提供一个液相的环境,使得各反应物的扩散系数提高,因此,其反应温度和反应时间均有明显下降[13-14]。但熔盐法处理铁尾矿作为一个尚未开拓的领域,急需开展大量的基础研究工作,而有关铁尾矿在熔盐体系中的分解动力学研究尚未见报道。针对这一问题,本文作者研究了反应温度及反应时间对铁尾矿在熔盐体系中分解率的影响,得到铁尾矿的分解动力学模型,这对铁尾矿熔盐清洁处理新工艺的设计具有重要的理论意义和实际意义。
1 实验
1.1 实验原料
实验所用主要原料是鞍山型铁尾矿(鞍山钢铁集团),其主要成分见表1。从表1可以看出,在铁尾矿中主要成分为SiO2及Fe2O3,其它微量成分为Al2O3、CaO、MnO和TiO2。颗粒的起始粒度及分布决定原料的反应活度,颗粒的累积分布如图1所示,由1图可知,铁尾矿的原始粒度为d50=12 μm。图2所示为未处理铁尾矿和熔盐煅烧铁尾矿的XRD谱。从图2(b)中可以看出,尾矿中主要物相是α-石英相及赤铁矿相,其它原料为NaOH(分析纯)和NaNO3(分析纯)。
表1 实验用铁尾矿主要成分
Table 1 Composition of iron ore tailings in experiment (mass fraction, %)
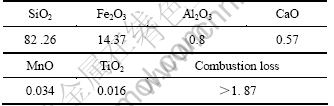

图1 铁尾矿的粒度累计分布图
Fig.1 Particle size distribution of iron ore tailings
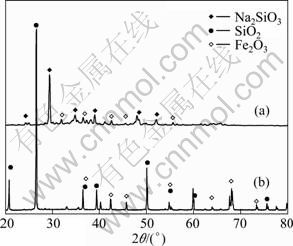
图2 未处理铁尾矿与熔盐煅烧铁尾矿的XRD谱
Fig.2 XRD patterns of as-received iron ore tailings (a) and iron ore tailings calcined by molten-salt method at 500 ℃ for 3 h (b)
1.2 实验过程
将NaOH和NaNO3及铁尾矿按照一定的摩尔比例混合均匀,然后将混合好的物料放至马弗炉中在不同条件下热处理,冷却到室温,球磨1 d。将熔盐处理的铁尾矿粉末按体积比1:5加入到去离子水在空气中搅拌陈化1 d,再经抽滤后得到硅酸钠溶液。经ICP-AEC测试得到溶液中Si的浓度,进而计算SiO2浸出率。SiO2浸出率X定义为
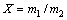 (1)
(1)
式中:m1为尾矿浸出到溶液中SiO2的质量;m2为未处理尾矿中SiO2的质量。
1.3 实验原理
ZHANG等[15]认为n(NaOH):n(NaNO3)=2.2:1熔盐体系的熔点为280 ℃,所以铁尾矿在NaOH-NaNO3熔点温度以上的反应过程是一个典型的液-固反应过程。图2所示为未处理铁尾矿与熔盐煅烧铁尾矿的XRD谱。由图2可知,经熔盐反应后铁尾矿中的SiO2转化为硅酸钠相,则铁尾矿在NaOH-NaNO3熔盐介质中反应可表示为
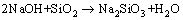 (2)
(2)
1.4 分析与表征
采用热电公司Iris Advangtage 1000 ICP-AEC测定溶液中元素含量。采用理学公司D/MAX-RB型X射线衍射仪进行物相组成分析。欧美克公司的LS900型激光粒度仪测试尾矿粒度。
2 结果与讨论
2.1 保温时间对SiO2浸出率的影响
图3所示为不同保温时间SiO2的浸出率。固定反应条件为n(NaOH):n(NaNO3):n(SiO2)= 2.2:1:1(摩尔比),煅烧温度500 ℃。由图3可知,随着保温时间的延长,SiO2浸出率增加,当反应时间小于180 min时,SiO2浸出率增加较快;当煅烧保温时间为180 min时,SiO2浸出率为88.42%;但当反应时间超过180 min时,白炭黑产率增加缓慢,300 min时才增加到91%左右,因此适宜的保温时间为180 min。
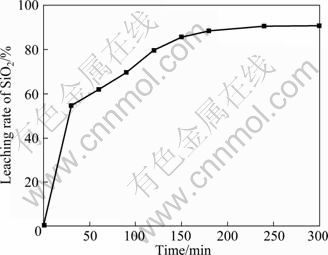
图3 不同保温时间的SiO2浸出率
Fig.3 Leaching rate of SiO2 after different keeping times
2.2 碱渣比对SiO2浸出率的影响
图4所示为不同的碱与渣中SiO2的摩尔比对SiO2的浸出率的影响,固定反应条件为n(NaOH):n(NaNO3)=2.2:1,煅烧温度500 ℃,保温时间180 min。由图4可以看出,SiO2的浸出率随NaOH与SiO2比值的增加而增加,当n(NaOH):n(SiO2)=2.2(摩尔比)时,SiO2的浸出率为88.42%;继续增加碱的加入量,虽然SiO2的浸出率有所增加,但增加趋势较缓,因此选择n(NaOH):n(SiO2)=2.2:1为最佳配比。根据反应式(1),SiO2与NaOH完全反应的理论质量比约为l:2,但铁尾矿中许多杂质均与氢氧化钠发生反应消耗一部分碱,所以适当增加碱量利于提高SiO2的浸出率。同时,反应体系内,NaOH-NaNO3处于熔融态,碱量增大时,整个体系的流动性增强,黏度降低,物料间的传质阻力降低,利于SiO2的深度转化。
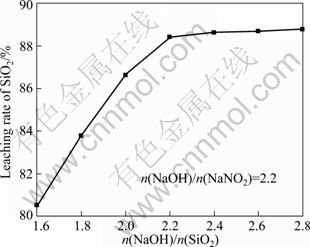
图4 NaOH和SiO2摩尔比对SiO2浸出率的影响
Fig.4 Effect of mole ratio of NaOH and SiO2 ratio on leaching rate of SiO2
2.3 煅烧温度对浸出率的影响
反应温度对化学平衡和反应速率有着重要的影响,因此煅烧温度对SiO2与NaOH反应转变为Na2SiO3有很大的影响。在n(NaOH):n(NaNO3):n(SiO2)= 2.2:1:1,煅烧保温时间为180 min条件下,选择不同温度进行实验,SiO2的浸出率如图5所示。
由图5可知,当煅烧处理温度高于400 ℃时,在体系中出现液相,发生熔盐作用,SiO2与NaOH开始进行反应生成Na2SiO3相。随着反应温度的不断升高,高温体系中的液相不断增多,增加了SiO2与NaOH反应的扩散系数,提高了反应的转化率。从图5曲线可以看出,随着煅烧温度的升高,SiO2的浸出率不断升高,温度到500℃时浸出达到极值,为88.42%,此时为最佳浸出温度。
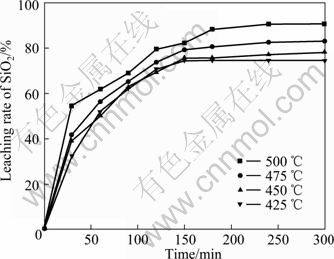
图5 不同温度下SiO2的浸出率
Fig.5 Leaching rate of SiO2 at different temperatures
2.4 浸出反应动力学模型
由于铁尾矿在NaOH-NaNO3熔盐介质中反应时有固体产物生成,因此可以认为反应过程适用于未反应收缩核模型,假定反应前后颗粒的体积变化可以忽略,整个反应由液相边界层的扩散、固态产物层内的扩散和界面化学反应3个步骤混合控制。由于液相边界层的扩散过程除了受溶液的物性影响之外,还取决于实际反应器中的速度场、温度场和浓度场,因此,外扩散过程与反应器的结构、是否有搅拌以及搅拌强度等因素有关。由于实验中所用的搅拌转速为1 000 r/min,同时反应器较小,因此可认为反应过程中已基本消除液相边界层的扩散的影响。 在实际浸出过程中,反应速率可能受固态产物层内的扩散或界面化学反应其中一步控制,其动力学方程如下所示[16-19]。
界面化学反应控制过程:
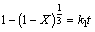 (3)
(3)
固体产物层内扩散控制过程:
 (4)
(4)
式中:t 为反应时间;X 为反应时间为 t 时SiO2浸出率;k1和k2分别为反应速率常数。
2.5 反应过程控制步骤的确定及反应活化能的确定
为确定n(NaOH):n(NaNO3):n(SiO2)= 2.2:1:1,铁尾矿平均粒径为12 μm时铁尾矿分解过程的控制步骤,利用图5中反应温度为425 ℃时的SiO2浸出率数据,计算出1+2(1-X)-3(1-X)2/3及1-(1-X)1/3,将其分别对反应时间t作图,根据各自线性相关系数(R′)的大小确定铁尾矿分解过程的控制步骤,其结果如图6所示。
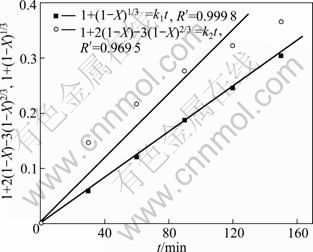
图6 425 ℃时1+2(1-X)-3(1-X)2/3及1-(1-X)1/3与反应时间的关系
Fig.6 Plots of 1+2(1-X)-3(1-X)2/3 and 1-(1-X)1/3 vs reaction time
从图6中可知,在NaOH-NaNO3熔盐与铁尾矿反应最快的阶段,1+2(1-X)-3(1-X)2/3与反应时间t的线性关系非常好,线性相关系数为0.999 8;1-(1-X)1/3与反应时间t的线性关系较差,仅为0.969 5,说明铁尾矿在NaOH-NaNO3熔盐介质中分解的初始阶段更符合固体产物层内扩散控制。
根据图5中不同反应温度的SiO2浸出率数据计算出1+2(1-X)-3(1-X)2/3,并将其与反应时间t作图,其结果如图7所示。
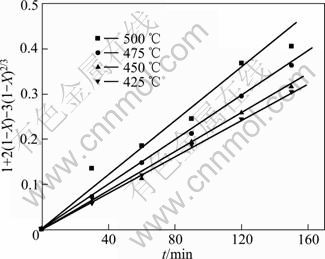
图7 1+2(1-X)-3(1-X)2/3与反应时间的关系
Fig.7 Plots of 1+2(1-X)-3(1-X)2/3 vs reaction time
由图7可以看出,在所研究的温度范围内,出1+2(1-X)-3(1-X)2/3与反应时间t之间呈良好的线性关系,这进一步说明了当n(NaOH):n(NaNO3):n(SiO2)= 2.2:1:1,铁尾矿平均粒径为12 μm时,铁尾矿在NaOH-NaNO3熔盐中的分解过程为固体产物层内扩散控制。由图7中各直线可以求出其斜率,各斜率分别代表不同反应温度下的反应速率常数(k)。根据阿伦尼乌斯方程:
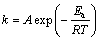 (5)
(5)
式中:A为常数;Ea为表观活化能。则有
 (6)
(6)
将lnk与1/T作图,其结果如图8所示。从图8可知,1nk与1/T呈直线关系,从该直线斜率可以求出该实验条件下反应的表观活化能,活化能计算式如下。
Ea=2 095.9R=17.43 kJ/mol (7)
进一步证明了铁尾矿在NaOH-NaNO3熔盐介质中的分解过程为固体产物层内扩散控制[20]。
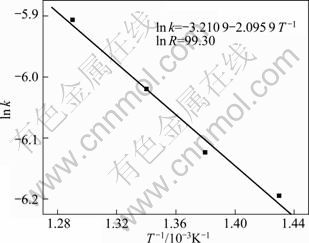
图8 lnk与1/T的关系
Fig.8 Plots of lnk vs 1/T
结合式(4)和(6)可知
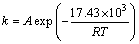 (8)
(8)
由图8中直线截距求出A值:
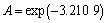 =0.040 3 min-1 (9)
=0.040 3 min-1 (9)
则在n(NaOH):n(NaNO3):n(SiO2)= 2.2:1:1,铁尾矿平均粒径为12 μm的实验条件下,铁尾矿在NaOH-NaNO3熔盐介质中反应的宏观动力学方程式为
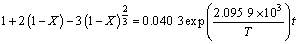 (10)
(10)
3 结论
1) 反应温度及反应时间均对SiO2在NaOH-NaNO3熔盐介质中浸出率有显著的影响,提高反应温度或者延长反应时间均能提高SiO2的浸出率。
2) SiO2在NaOH-NaNO3熔盐介质中的浸出过程符合未反应收缩核模型,为固体产物层内扩散控制,反应活化能为17.43 kJ/mol。
3) 在n(NaOH):n(NaNO3):n(SiO2)= 2.2:1:1,铁尾矿平均粒径为12 μm的实验条件下,铁尾矿在NaOH-NaNO3熔盐介质中反应的宏观动力学方程式为
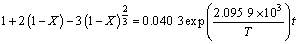 。
。
REFERENCES
[1] ZHANG S, XUE X, LIU X, DUAN P, YANG H, JIANG T, WANG D, LIU R. Current situation and comprehensive utilization of iron ore tailing resources [J]. J Min Sci, 2006, 42(6): 403-408.
[2] MATSCHULLAT J, BORBA P R, DESCHAMPS E. Human and environmental contamination in the iron quadrangle[J]. Appl Geochem, 2000, 15(2): 193-202.
[3] 张淑会, 杨 合, 薛向欣, 王东山. 原料组成和氩气流量对铁尾矿合成SiC粉末的影响[J]. 东北大学学报: 自然科学版, 2006, 27(10): 1114-1117.
ZHANG Shu-hui, YANG He, XUE Xiang-xin, WANG Dong-shan. Effect s of raw materials composition and Ar flux on SiC powder synthesized from iron ore tailings [J]. Journal of Northeastern University: Natural Science, 2006, 27(10): 1114-1117.
[4] 徐凤平, 周兴龙, 胡天喜. 我国尾矿资源利用现状及建议[J]. 云南冶金, 2007, 36(4): 25-27.
XU Feng-ping, ZHOU Xing-long, HU Tian-xi. Current situation of using tailing resource in our country and some suggestions [J]. Yunnan Metallurgy, 2007, 36(4): 25-27.
[5] 闫满志, 白丽梅, 张云鹏, 张锦瑞. 我国铁尾矿综合利用现状问题及对策[J]. 矿业快报, 2008(7): 9-13.
YAN Man-zhi, BAI Li-mei, ZHANG Yun-peng, ZHANG Jin-rui. Current situation, problems and countermeasures of comprehensive utilization of iron tailings in China [J]. Express Information of Mining Industry, 2008(7): 9-13.
[6] 张淑会, 薛向欣, 金在峰. 我国铁尾矿的资源现状及其综合利用[J]. 材料与冶金学报, 2004, 3(4): 9-13.
ZHANG Shu-hui, XUE Xiang-xin, JIN Zai-feng. Current situation and comprehensive utilization of iron ore tailings resources in our country [J]. Journal of Materials and Metallurgy, 2004, 3(4): 9-13.
[7] 王运敏, 常前发. 当前我国铁矿尾矿的资源状况利用现状及工作方向[J]. 金属矿山, 1999, 271(1): 1-6.
WANG Yun-min, CHANG Qian-fa. The present conditions of China’s iron tailings resource and the art-of-state and effort direction of its utilization [J]. Metal Mine, 1999, 271(1): 1-6.
[8] 雷 力, 周兴龙, 李家毓, 蔡晓威, 王国文. 我国矿山尾矿资源综合利用现状与思考[J]. 矿业快报, 2008(9): 5-8.
LEI Li, ZHOU Xin-long, LI Jia-min, CAI Xiao-wei, WANG Guo-wen. Status quo and pondering on comprehensive utilization of mine tailings resources in China [J]. Express Information of Mining industry, 2008(9): 5-8.
[9] DAS S K, KUMAR S, RAMACHANDRARO P. Exploitation of iron ore for the development of ceramic tiles [J]. Waste Manage, 2000, 20(8): 725-729.
[10] RANE K S, VERENKAR V M S, SAWANT P Y. Dielectric behaviour of MgFe2O4 prepared from chemically beneficiated iron ore rejects [J]. Bull Mater Sci, 2001, 24(3): 323-330.
[11] SAKTHIVEL R, DAS B, SATPATI B. Gold supported iron oxide–hydroxide derived from iron ore tailing for CO oxidation[J]. Appl Surf Sci, 2009, 255(13/14): 6577- 6581.
[12] LI J, WANG Q, LIU J. Synthesis process of forsterite refractory by iron ore tailings[J]. J Environ Sci, 2009, 21(Suppl 1): s92-s99.
[13] KUMAR P, MAL N, OUMI Y. Mesoporous materials prepared using coal fly ash as the silicon and aluminum source[J]. J Mater Chem, 2001, 11: 3285-3290.
[14] 于洪浩, 薛向欣, 贺燕, 黄大威. 熔盐法从铁尾矿中制取高纯白炭黑[J]. 化工学报, 2009, 60(8): 2124-2129.
YU Hong-hao, XUE Xiang-xin, HE Yan, HUANG Da-wei. Preparation of high purity precipitated silica from iron ore tailings by molten salt method [J]. Journal of Chemical Industry and Engineering, 2009, 60(8): 2124-2129.
[15] ZHANG Y, ZHENG S, XU H, DU H, ZHANG Y. Decomposition of chromite ore by oxygen in molten NaOH-NaNO3[J]. Int J Miner Process, 2010, 95(1/4): 10-17.
[16] 朱炳辰. 化学反应工程[M]. 北京: 化学工业出版社, 1993: 223.
ZHU Bing-chen. Chemical reaction engineering[M]. Beijing: Chemical Industry Press, 1993: 223.
[17] 蒋汉瀛. 湿法冶金过程物理化学[M]. 北京: 冶金工业出版社, 1984: 89-100.
JIANG Han-ying. Physical chemistry of hydrometallurgical processes [M]. Beijing: Metallurgical Industry Press, 1984: 89-100.
[18] SEHILM J, HERRINANN M, MIEHAEL G. Kinetic study of the corrosion of silicon nitride materials in acids [J]. J Eur Ceram Soc, 2003, 23(4): 577-584.
[19] WEISENER C G, SMART R, GERSON A R. Kinetics and mechanisms of the leaching of low Fe sphalerite [J]. Geochim Cosmochim Acta, 2003, 67(5): 823-830.
[20] SAIKIA N, SENGUPTA P, GOGOI P K. Kinetics of dehydroxylation of kaolin in presence of oil field effluent treatment plant sludge [J]. Appl Clay Sci, 2002, 22(3): 93-102.
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(21043007)
收稿日期:2010-10-26;修订日期:2011-03-11
通信作者:于洪浩,讲师,博士;电话: 024-23680841; E-mail: yuhh_fshs@yahoo.com.cn