一种新的非电解法炼铝工艺
来源期刊:中国有色金属学报(英文版)2016年第9期
论文作者:Oleg G. PARFENOV Andrey D. KUSTOV Leonid A. SOLOVYOV
文章页码:2509 - 2517
关键词:铝;铝合金;氯化物;碘化物;锌;碘;高速冶金
Key words:aluminum; aluminum alloy; chlorides; iodides; zinc; iodine; high-speed metallurgy
摘 要:提出了一种从氧化铝中提取原铝的新方法。该方法的原理基于如下化学反应:AlI3+(3/2)Zn=Al+(3/2)ZnI2。首先通过氧化铝的碳热氯化反应得到氯化铝(AlCl3),然后通过氯化铝和碘化钙的交换反应(AlCl3+CaI2→ AlI3+CaCl2)得到碘化铝。在实验室条件下对这些反应进行了研究,同时对一些回收主要化学试剂的反应进行了研究(Cl2, ZnI2→Zn, CaCl2→CaI2)。采用XRD和SEM对反应产物的相组成及形貌进行分析。采用总自由能最小方法对化学反应平衡进行计算。结果表明,采用非电解方法,可以在没有非常高的温度和可消耗的化学试剂的条件下,从氧化铝中有效地提取铝。与当今采用燃煤发电提供电力的铝电解厂相比,本方法所产生的单位碳消耗和CO2大气排放可以降低一半以上。
Abstract: A new method of primary aluminum extraction from alumina was proposed. The method is based on the new reaction AlI3+(3/2)Zn=Al+(3/2)ZnI2. In its turn, the exchange reaction AlCl3+CaI2→AlI3+CaCl2 is used to get aluminum iodide from aluminum chloride—the product of alumina carbochlorination. These reactions were studied in laboratory experiments as well as additional reactions, which were needed for the main chemicals recycling: Cl2, ZnI2→Zn, CaCl2→CaI2. XRD and SEM methods were used to investigate the phases and morphology of the reaction’s solid products. The global free energy minimization method was used for the chemical equation’s calculations. It was shown that aluminum can be effectively extracted from alumina without electrolysis, extreme high temperature and expended chemicals. The estimated specific carbon consumption and CO2 atmospheric pollution rate have to be halves of such values for the contemporary aluminum plant powered by the coal power station.
Trans. Nonferrous Met. Soc. China 26(2016) 2509-2517
Oleg G. PARFENOV, Andrey D. KUSTOV, Leonid A. SOLOVYOV
Institute of Chemistry and Chemical Technology of SB RAS, Akademgorodok 50/24, Krasnoyarsk 660036, Russia
Received 21 August 2015; accepted 30 May 2016
Abstract: A new method of primary aluminum extraction from alumina was proposed. The method is based on the new reaction AlI3+(3/2)Zn=Al+(3/2)ZnI2. In its turn, the exchange reaction AlCl3+CaI2→AlI3+CaCl2 is used to get aluminum iodide from aluminum chloride—the product of alumina carbochlorination. These reactions were studied in laboratory experiments as well as additional reactions, which were needed for the main chemicals recycling: Cl2, ZnI2→Zn, CaCl2→CaI2. XRD and SEM methods were used to investigate the phases and morphology of the reaction’s solid products. The global free energy minimization method was used for the chemical equation’s calculations. It was shown that aluminum can be effectively extracted from alumina without electrolysis, extreme high temperature and expended chemicals. The estimated specific carbon consumption and CO2 atmospheric pollution rate have to be halves of such values for the contemporary aluminum plant powered by the coal power station.
Key words: aluminum; aluminum alloy; chlorides; iodides; zinc; iodine; high-speed metallurgy
1 Introduction
All primary aluminum (53.127 MT in 2014) plants in the world make use of electrolysis now. The electricity consumption of aluminum plants in 2014 was 690170 GW·h, including 400572 GW·h produced by the coal power stations [1]. Typical efficiency of the heat to electricity conversion value is ~35%. If the electricity consumption for electrolysis is 13 kW·h per 1 kg Al, the higher heating value of the carbon is 32.79 MJ/kg (94.05 kcal/mol [2]), and carbon expenditure for the aluminum smelter anode production is 0.5 kg per 1 kg Al, the total carbon consumption is 4.58 kg per 1 kg Al. Total CO2 emission of the Coal Power Station and Aluminum Plant (CPSAP) pair was 892 MT in 2014.
It will be shown in this paper that the coal consumption and the carbon dioxide atmospheric emission can be reduced by more than halves with the help of the new method of aluminum production. No electricity, no extreme high temperature or pressure, no consumable chemicals, but carbon or coal, and oxygen or air are needed for this method. Moreover, the CPSAP can become both an aluminum producer and an electrical current supply for the outer consumer. If the steam-turbine cycle of the modern power station will be replaced by the couple “Al-air battery” [3], or “Al- iodine cell” (see below), and the apparatus for Al2O3→Al, or AlI3→Al reduction, power station will turn into coal-air fuel cell, which transforms the heat energy of combustion into electricity without Carnot limitations.
2 Brief review of known methods for aluminum reduction
Metallurgy of aluminum has been given rise in the first part of the 19th century, when the first powerful electrical energy source had been created. In that time aluminum was produced with the help of reduction of the aluminum chloride by alkali metals. Alkali metal production needs electrolysis. In the end of 1870s the cost of aluminum had been reduced to the cost of silver [4]. Hall-Heroult process gave an opportunity to reduce the aluminum cost by 7.5 times. In 2015 (June) the aluminum to silver cost relation was about 1:300. It is high enough as well. In the beginning of the 21st century the iron (steel) to primary aluminum annual mass production relation was ~20:1. The main causes of the relatively small aluminum production are its small consumption due to high price, and limitation of the total power stations capacity [3].
Along with the electrolysis technology, non-electrolytic methods of aluminum reduction were proposed: direct carbothermal reduction processes (DCRPs) (1/2)Al2O3+(3/2)C→Al+(3/2)CO, and indirect carbothermal reduction processes (ICRPs) [5,6]. DCRPs are not used due to extreme high temperature (more than 2273 K (2000 °C)), needed for the reduction’s high yield. For example, the DCRP’s equilibrium thermodynamics yield that is higher than 90%, is permitted at >2600 °C. ICRP uses several chemical transformations (two stages as a rule) and intermediate compounds for the Al-reduction. It replaces the direct Al-reduction from Al2O3 by its reduction from intermediate compounds: AlCl3, AlN, and so on, by electrolysis (AlCl3→Al (cathode)+1.5Cl2 (anode)) or thermal decomposition at high temperature (AlN→Al+0.5N2, >2707 K (2434 °C) at 0.1 MPa). Very high temperature is needed for the ICRP [7,8] as well.
3 Main idea
Aluminum reduction temperature can be decreased to 573-723 K (300-450 °C). The new method is based on the principles of the high-speed metallurgy (HSM):
1) No expensive consumable chemicals;
2) No waste products except for CO2;
3) No bulk electrical energy consumption;
4) Universal technology approach and apparatus for multicomponent ores processing;
5) Rejection of a chemical reaction, if it is characterized by the low yield, very high temperature, extreme high or low pressure, and if it can be substituted by a set of chemical reactions, that are not characterized with such peculiarities.
Practical significance of Points 1, 2 is obvious. Point 3 has a great importance for the aluminum production because of strict limitation of electric energy in some industrial regions of the world.
Universal technology (Point 4) is needed to process multicomponent ores. These ores contain a main body of the total mineral reserves. Up to now most of metallurgical plants can process the monocomponent ores or concentrates only.
HSM includes three main stages: halogenations of the multicomponent feedstock, separation and purification of the halides, and reduction of metals from the halides.
Point 5 is the principal point for the new method: DCRP is substituted by 10 stages (vide infra). Earlier, we have used this approach to replace well known catalytic one stage Deacon Process (HCl→Cl2) and two stages of the Klaus Process (H2S→S) by the HSM processes. These catalytic processes are characterized by the low equilibrium yield. We have increased their theoretical (and practical as well) yields by replacing catalytic reactions with a set of non-catalytic chemical transformations [9,10]. Partition of the chemical reactions gives an opportunity to evade thermodynamic limitations. In Ref. [11] we have used the thermodynamic calculations to prove this statement. DCRP was separated into the five stages: Al2O3→AlI3→Al-Zn alloy→Al; ZnI2→ZnO→Zn. Zinc was proposed as the reducing agent due to thermochemical properties of the ZnI2(s). But impossibility to get the high yield of the carboiodination ((1/2)Al2O3(s)+(3/2)C(s)+(3/2)I2(g)= AlI3(g)+(3/2)CO(g)) has been predicted. Our subsequent experiments have confirmed this prediction.
A new set of chemical reaction is proposed to evade carboiodination problem by the carbochlorination (Al2O3→AlCl3) and exchange (AlCl3→AlI3) reactions:
(1/2)Al2O3(s)+(3/2)C(s)+(3/2)Cl2(g)=AlCl3(g)+(3/2)CO(g), T=1573 K (1)
AlCl3(g)+(3/2)CaI2(s)=AlI3(g)+(3/2)CaCl2(s),
T=773-823K (2)
AlI3(l)+(x+(3/2))Zn(s)=Al-Znx(s)+(3/2)ZnI2(s),
T=613 K (3)
Al-Znx(s)=Al(l)+xZn(c), T=1193 K (4)
(3/2)CaCl2(l)+(3/2)SiO2(s)+(3/4)O2(g)=(3/2)Cl2(g)+(3/2)CaSiO3(s), T=1473 K (5)
(3/2)CaSiO3(s)+3HI(aq)=(3/2)CaI2(aq)+(3/2)SiO2(s)+(3/2)H2O(l), T=373 K (6)
(3/2)C(s)+(3/2)H2O(g)=(3/2)CO(g)+(3/2)H2(g),
T=1273 K (7)
(3/2)I2(g)+(3/2)CO(g)+(3/2)H2(g)=3HI(g)+(3/2)CO(g), T=773-1073 K (8)
(3/2)ZnI2(g)+(3/4)O2(g)=(3/2)ZnO(s)+(3/2)I2(g),
T=873-973 K (9)
(3/2)ZnO(s)+(3/2)C(s)=(3/2)Zn(g)+(3/2)CO(g),
T=1193-1273 K (10)
Block diagram for the HSM method of aluminum reduction is shown in Fig. 1. The Reactions (1)-(3) describe the principal chemical transformations, Reactions (5)-(10) are the auxiliary reactions, and stage 4 is the physical purification of Al. The aim of auxiliary reactions is the chlorine, calcium iodide, and zinc recycling. Optimal temperatures for Reactions (1)-(10) are calculated with the method of total Gibbs energy minimization, used in Ref. [12]. Gas pressure 0.1 MPa in the chemical reactors was used for each chemical reaction. Stage 4 used vacuum as a rule.
Generalized reaction (GR) (1/2)Al2O3(s)+(9/2)C(s)+ (6/4)O2(g)=Al(l)+(9/2)CO(g) is the sum of the right and left parts of Eqs. (1)-(10).
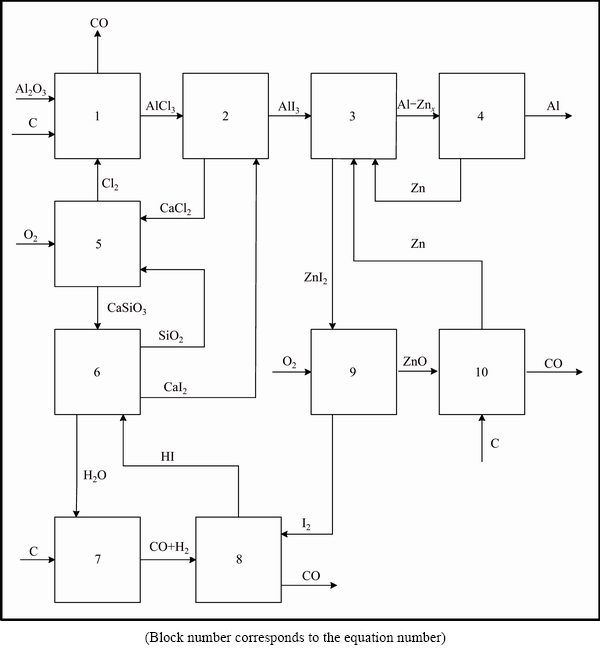
Fig. 1 Block diagram of high-speed aluminum metallurgy
4 Equipment
The powder X-ray diffraction (XRD) measurements were carried out by using the PANalytical X'Pert PRO diffractometer with Co Kα radiation. The quantitative phase analysis was performed using the full-profile Rietveld method [13] and the derivative difference minimization technique [14]. The mass fractions of crystalline phases Wi and amorphous residue Wr were calculated as follows:
Wi=μSiMiViNi2/Ct, Wr=1-μWi
where Si is the refined scale factor of phase i, Mi and Vi are the unit cell mass and volume, Ni is the space group multiplicity, μ is the mass absorption coefficient, C is the diffractometer constant determined from an external standard (corundum) measurement, and t is the scan counting time.
The equilibrium calculations of the chemical compositions were made with the global free energy minimization (FEM) method [12]. Images of zinc powder and Al-Zn sponge were made with the electron scanning microscope (SEM, Hitachi TM1000).
5 Results and discussion
Reaction (1) has been well studied and utilized in laboratories and plants. It is the slowest and most difficult process among the Reactions (1)-(10). It needs about 3 h, temperature of 1573 K (1300 °C) and a chlorine/chloride resistant material (corundum) for the reactor’s tube (in this work we have used the method and apparatuses described in Ref. [12]). Chlorine, metallurgical alumina, and demineralized coal were used. High temperature was needed for the best kinetics due to high α-Al2O3 (~40%) content. Thermodynamics yield Y1 is about 99.9%. For γ-Al2O3 the carbochlorination temperature can be reduced to 1073-1173 K (800-900 °C) [15]. To reduce the time of carbochlorination, the admixture of 5%-10% KCl or NaCl can be used.
Reaction (2) is needed to transform aluminum chloride into aluminum iodide. Thermodynamics yield Y2=[AlI3]out/[AlCl3]in (for the NIST and NASA thermo- chemical data, used in Ref. [12]) in equilibrium conditions is 25%. For the data in Ref. [16] this value is 50%. Here [AlCl3]in and [AlI3]out are the moles of aluminum halides before and after Reaction (2), respectively. Low yield problem can be solved with the help of non-equilibrium conditions in a reactor, where aluminum halides are used as gas flows, and calcium halides as solid substances. In this work Reaction (2) was studied in the tube furnace (Fig. 2) at T=773 K (500 °C) in Ar flow (0.06 L/min). Porous granules (~5 mm) of the anhydrous CaI2 were put into tube (80 mm filling length, 16 mm diameter). Product (AlI3) was collected in the cooler. It was found that Y2=73%. This value is much higher than the thermodynamic equilibrium value. Y2 may be increased with the help of the reaction’s zone length increasing.
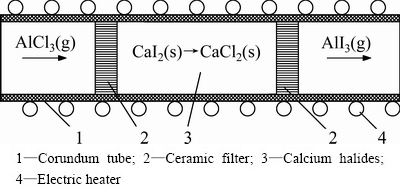
Fig. 2 Scheme of reactor for exchange Reaction (2)
There are peculiarities of Reaction (2) carrying out: 1) temperatures have to be less than the melting points
of calcium halides (1045 K (772 °C) for CaCl2, 1056 K (783 °C) for CaI2 [2]) and the double salt of xCaCl2·yCaI2 (831 K (558 °C) for x=0.63, y=0.37 [17]) to prevent surface contraction due to salts melting; 2) temperatures have to be higher than the sublimation/ boiling points of aluminum halides: 453 K (180 °C) for AlCl3, and 655.65 K (382.5 °C) for AlI3 [2].
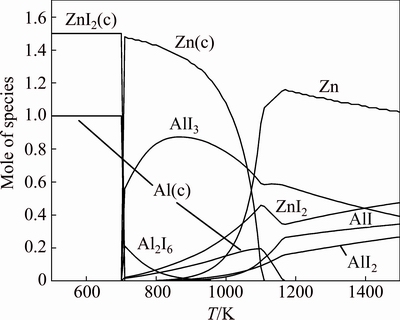
Fig. 3 Equilibrium molar composition of products of reactions in AlI3+(3/2)Zn mixture at P=0.1 MPa ((c)–condensed phase) [11]
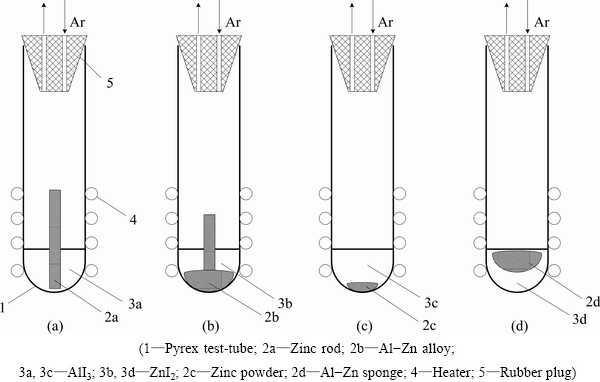
Fig. 4 Scheme for Reaction (3)
Reaction (3) is the principal one in high-speed aluminum metallurgy. Thermodynamic calculations (Fig. 3) predict the yield of the reaction Y3=[Al]/[AlI3]= 100% [11] at temperature below the melting point of zinc iodide (719 K (446 °C) [2]). Relatively low temperature is useful for the industrial production. Conversely, it provides the problems of the removing of the solid reaction products (Al(s), ZnI2(s)) continuously during the chemical transformations. Several variants were studied to solve this problem. The couple of them are described in this work (Fig. 4): 1) zinc rod and aluminum iodide (Tmelt=461.45 K (188.3 °C)) were put into glass test-tube, and heated up to temperature T (654 K
The first variant (Figs. 4(a) and (b)) is the simplest one for the practice. It is based on Al-Zn alloy melting diagram (Fig. 5). At T=654 K (381 °C) melting started at the mole relation of [Al]:[Zn]=11.3:88.7 (the mass relation is 5:95). The higher the temperature, the more this relation. Reduced aluminum in the form of Al-Zn alloy drops fall down to the test-tube bottom. No pores, no problem with salt admixture in this alloy (see below). But it takes a plenty of heat energy for the zinc evaporation from this drop afterwards. It is the main disadvantage of the first variant. Pure zinc evaporation enthalpy is ΔH=27.44 kcal/mol=1.75 MJ/kg [2]. It needs more than 33 MJ of heat energy to extract 1 kg of aluminum from Al-Zn alloy (for Al 5%, mass fraction). Another disadvantage is the slow reaction rate due to the small specific surface of the Zn-AlI3 contact.
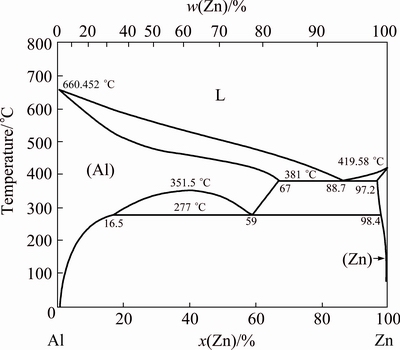
Fig. 5 Al-Zn phase diagram [18]
In the second variant (Figs. 4(c) and (d)) the mixture of AlI3 and Zn-powder was transformed into the Al-Zn sponge (Al 80%) (Fig. 6(b)). The density of this sponge was about 1 g/cm3. It means that the obtained product had a lot of pores. Temperature (613 K (340 °C)) was chosen to be less than AlI3 boiling point, and Al-Zn alloy melting point. It needs distillation (Block 4, Fig. 1) to produce the pure aluminum from this sponge.
The second variant’s advantages are: zinc powder is obtained in Reaction (10) and no additional working over is needed, the process takes a few minutes, the experimental aluminum yield Y4=[Al]/[AlI3] is above 90%, and the zinc content in sponge alloy (Fig. 7) is small enough compared to the first variant.
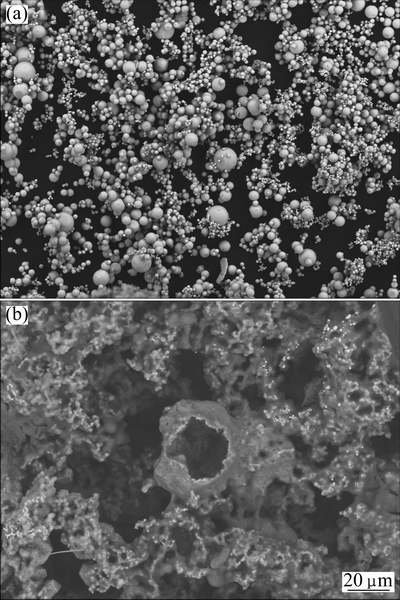
Fig. 6 SEM images of zinc powder as product of Reaction (10) (a), and Al-Zn sponge as product of Reaction (3) at T= 613 K (340 °C) and P=0.1 MPa (b)
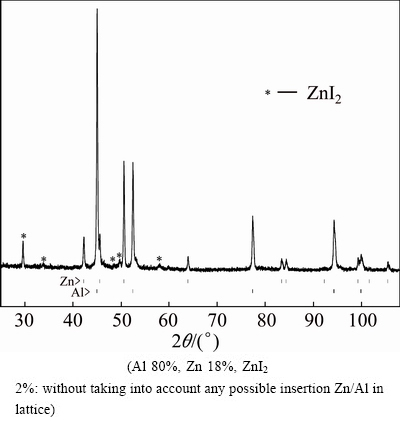
Fig. 7 XRD pattern of Al-Zn sponge
Reactions (7) and (8) are required for the HI acid synthesis. The corundum tube reactor was used (Fig. 8). Synthesis of CO+H2 gas mixture (Reaction (7)) is the well-studied process. Steam flow was 0.12 g/min. Up to now on practice the catalytic method of HI synthesis by element combining (I2+H2→2HI) was used [19]. In our case, this reaction has taken place in the upper part of reactor. Carbon monoxide is an inert gas in HI synthesis, so CO does not need to be removed from the synthesis gas. Afterwards CO can be used for the additional heat energy production (see below). Hydrogen iodide was absorbed by the water to produce hydroiodic acid solution. Excess water was evaporated up to 50% acid concentration, and acid was used in Reaction (6) afterwards. The combining of Reactions (7, 8) in one reactor was possible after the coal demineralization in 20% HCl solution at 373 K (100 °C), 2 h.
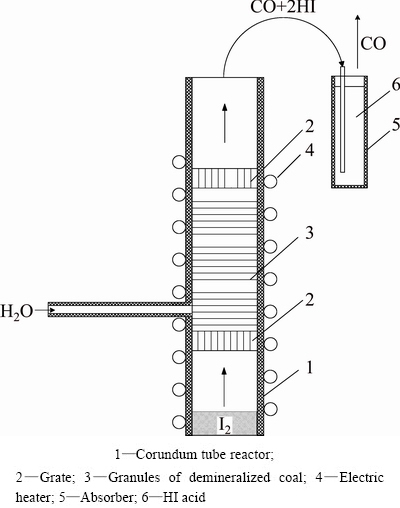
Fig. 8 Scheme of HI acid synthesis
It must be mentioned that the large scale coal demineralization process needs the full acid regeneration. HBr acid is more preferable for this task [20] because of the high calcium content in the coal ash.
Reaction (5) is needed for the chlorine extraction from CaCl2 to return Cl2 to the carbochlorination stage (Block 1, Fig. 1). Earlier more complex method for this task was used—a sintering of calcium chloride with kaolin clay in the air atmosphere. Anorthite (CaAl2Si2O8) and chlorine were the products of this sintering [21]. But kaolin clays (and bauxites as well) are the perspective raw materials for the high-speed aluminum metallurgy [12]. In this work, another approach was proposed—a sintering of calcium chloride with silica in the air atmosphere. Pseudowollastonite (CaSiO3) and chlorine were the products of this sintering (Fig. 9). To prevent the surface area reduction due to CaCl2 melting, an additional silica powder ([СaSiO3]:[SiO2]=1) was added as inert material. This SiO2 admixture did not influence the chemical transformations in Reactions (5) and (6).
It was found that chlorine was extracted after sintering of calcium chlorine and silica mixture in 1 h at 1073 K (800 °C) in the air atmosphere. At the end of sintering temperature was increased up to 1473 K (1200 °C) to improve the yield. CaCl2—residue of 1%-2% was found in the products afterwards. It may be proposed that this residue is the result of the pseudowollastonite crust formation on the CaCl2 particles, and it will be reduced by using of rotary reactor with the inert grinding bodies for this crust crushing.
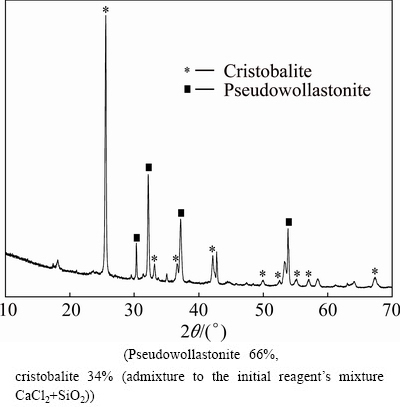
Fig. 9 Products of Reaction (5)
Reaction (6) is needed for calcium iodide regeneration. It takes 30 min at 373 K (100 °C) to process pseudowollastonite powder (<0.074 mm) by 50% HI acid into the CaI2 solution and SiO2 solid residue.
Reaction (9) is used for iodine recycling. Our experiments have shown that temperature had to be higher than 873 K (600 °C) (the ZnI2 vapor pressure is 0.1 MPa at 869 K (596 °C) [22]) for the fast reaction rate. Air is used as oxidizing agent. Main difficulty for the practice will be to entrap fine (less than 1 μm) particles of the zinc oxide. Perhaps, electrostatics traps have to be used for it at temperature higher than iodine boiling point. On the laboratory scale we have used the common trap for the ZnO, and I2 particles.
Reaction (10) was studied in the quartz tube reactor (Fig. 10). The initial mixture of zinc oxide powder (0.04-0.07 mm) and demineralized coal (0.04-0.07 mm) ([C]:[ZnO]=2:1) was placed into reactor and heated up to 1173-1273 K (900-1000 °C). It was found that no high temperature (1373-1573 K [23]) is needed for the zinc reduction. Zinc vapor appeared at temperature a bit less than 1173 K (900 °C) in argon flow. A nozzle was used for the fast adiabatic cooling of the gas mixture CO(CO2)+Zn(g) to prevent the reverse reaction.
Reaction (4) describes the removing of Zn from the Al-Zn alloy by the Zn evaporation. The best result of such process mentioned in scientific literature is 99.86% Al from Al-Zn alloy [24]. It takes 40 min at temperature of 1073 K (800 °C) and pressure of 18 Pa. In our experiments we used P=10 Pa and T=973 K (700 °C). The aluminum purity was higher than 99.5% (by SEM Hitachi TM-1000). Better aluminum purity was not the task of our investigation in this work. Al-Zn alloys are widely used for preparing more complex alloys in aircrafts manufacturing. As to Al-air battery it may be concerned that the problem of Zn-admixture in the aluminum electrodes demands spatially investigations as it was described in Ref. [25].
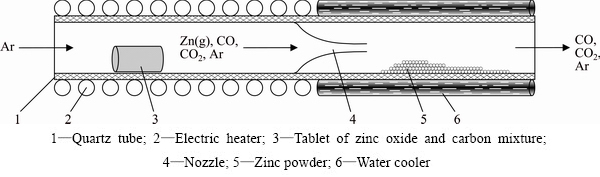
Fig. 10 Scheme of zinc reduction’s reactor
6 Specific productivity, energy estimations and some remarks
Beyond doubts, electrolysis will be dominated technology for the aluminum production during the nearest decades, in spite of its disadvantages: 1) limitation of the electrical energy sources; 2) dependence on decreasing of water resources for the hydroelectric power production; 3) low efficiency of electrical energy conversion into the metal—about 50% of electrical energy of aluminum smelter transforms into a dissipating heat; 4) ecological problems, which are connected with the low volume productivity of the electrolysis systems. It is well known that the specific volume productivity of Al of the smelters is 0.115 kg/(m3·h)—it is a result of anode–cathode distance of 0.05 m, and the anode surface productivity is 2.3 kg/(m2·h). As the result of the such low specific aluminum productivity, a huge working area is needed for the smelters and a lot of efforts are required to prevent air pollution by the electrolyte’s and anode’s compounds. A HSM – reactor for the aluminum reduction by Zn-powder (Fig. 3(c)) has the estimated specific aluminum productivity of about 1 kg/(cm3·h) or 1 t/(m3·h). To date it is impossible to make the more correct estimations of the full specific volume productivity of the ten stages (Fig. 1) on the base of preliminary laboratory experimental data only.
Each block (Fig. 1) is characterized by the temperature T in the reaction zone, and the reaction’s enthalpy ΔH —difference between enthalpies of initial reagents and products at constant T and pressure P=0.1 MPa. These values are presented in Table 1. For simplicity we accept that the yield of each reaction equals 100%; the enthalpy of Al-Zn alloy formation in Reaction (3) equals zero; data for Reaction (4) were calculated for alloy Al-80%, Zn-20%; ΔH for Reaction (6) was calculated at the normal conditions in the water solution; enthalpy of exothermic reaction (5) was excluded because of the absence of any reliable data in thermochemical database and scientific literature for this reaction.
Table 1 Enthalpies of Reactions (1-10)
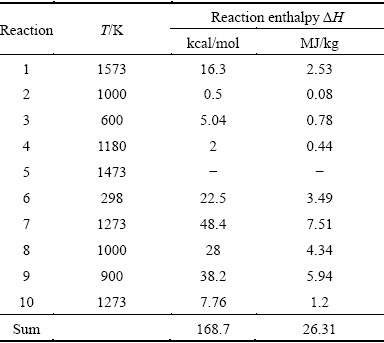
Carbon consumption per 1 kg Al, to support the reaction enthalpies 2631 MJ, is 0.8 kg. If we submit that heat utilization efficiency has to be 80%, the coal consumption has to be increased by 1.25 times. In this case carbon consumption to support the endothermic reaction enthalpies will be 1 kg per 1 kg Al. This value has to be increased by 2 kg due to the reagent expenditure (С→CO) in Reactions (1), (8) and (10). But carbon monoxide (co-product in these reactions) has to be used as a fuel for endothermic reactions temperature supporting. The enthalpy of reaction CO+1/2O2 →CO2 is -67.6 kcal/mol at standard conditions [2]. It decreases the total carbon consumption by 1.57 kg. This value is by 2.9 times less than that in traditional electrolysis technology. No doubts, this relation will have the less value in a practice due to heat losses, and so on.
A new method has some disadvantages from the traditional technology’s point of view. In addition to the mentioned above plenty of stages, another disadvantage of the new method is the heavy mass of the reagent’s and product’s flows due to very high iodine specific mass, and its high price ~40 US$/kg [26]. If we permit the iodine loss 1 g per 1 kg Al, the money loss will be about 2% of the current price of aluminum (~1750 US$/t). Annual iodine production in 2014 was ~35000 t [26]. It means that iodine supply is characterized by sufficient quantity for the non-electrolytic aluminum production or regeneration in fuel cells.
On the other hand, the high density of iodine and iodide vapors gives an opportunity to hold vapors in a local zone in vertical chemical reactors (Fig. 4). Furthermore, iodine is the convenient chemical reagent compared to fluorine, chlorine, and bromine. It is solid at normal conditions and quite safe for the storage and transportation.
Another advantage of iodine is the possibility to simplify scheme (Fig. 1) for direct heat to electricity conversion by the Al-iodine cell (reaction Al+(3/2)I2→ AlI3 [27]). Aluminum and iodine recycling needs Blocks 3, 4, 9 and 10 only.
In this work we have used rather expensive raw material—metallurgical alumina for the aluminum production. HSM gives an opportunity to extract aluminum as AlCl3 from any non-alkali ores with the help of carbochlorination [12]. Due to this possibility no metallurgical alumina plants are needed for processing bauxite, kaolin, or kyanite ores.
The most interesting application of the new method is direct aluminum and electricity production from coal. There are deposits and users of such grade coal. For example, there are consumers of coal which has abundant quantity of alumina (>38%) in ash in Inner Mongolia and Shanxi Province in China [28].
7 Conclusions
1) No electrolysis, no extreme high temperature (>1573 K (1300 °C)), no consumable chemicals, but carbon/coal and oxygen/air, are needed for the new method of aluminum production—the High Speed Aluminum Metallurgy.
2) High-speed aluminum metallurgy permits to save carbon fuel by more than half compared to modern aluminum plants that are powered by the coal power stations.
3) Formally the new method may be referred to the indirect carbothermal reduction processes with the generalized reaction (1/2)Al2O3(s)+(9/2)C(s)+ (6/4)O2(g)=Al(l)+(9/2)CO(g). The set of plenty stages is needed to overcome Al2O3→Al Gibbs energy barrier without electrolysis, alkali metals or plasma.
Acknowledgements
This investigation was supported by the Russian Academy of Science under project No. V.46.1.4 “High speed metallurgy”.
References
[1] The International Aluminium Institute. Statistics. 2014 [EB/OL]. [2015-10-30]. http:// www.world-aluminium.org/statistics/.
[2] GLUSHKO V P, MEDVEDEV V A. Thermochemical constants of substances. Parts 1 to 10 [M]. Moscow: Academy of Sciences, 1965-1981.
[3] WANG H, LEUNG D Y C, LEUNG M K H. Energy analysis of hydrogen and electricity production from aluminum-based processes [J]. Applied Energy, 2012, 90: 100-105.
[4] BINCZEWSKI G J. The point of a monument: A history of the aluminum cap of the washington monument [J]. JOM, 1995, 47(11): 20-25.
[5] RHAMDHANI M A, DEWAN M A, BROOKS G A, MONAGHAN B J, PRENTICE L. Alternative Al production methods: Part 1—A review of indirect carbothermal routes [J]. Mineral Processing and Extractive Metallurgy (Trans Inst of Mining and Metallurgy, Section C), 2013, 122(2): 87-104.
[6] DEWAN M A, RHAMDHANI M A, BROOKS G A, MONAGHAN B J, PRENTICE L. Alternative Al production methods: Part 2—Thermodynamic analyses of indirect carbothermal routes [J]. Mineral Processing and Extractive Metallurgy (Trans Inst of Mining and Metallurgy, Section C), 2013, 122(2): 113-121.
[7] KARTAYEV E V, KUSTOV A D, LUKASHOV V P, MICHALCHENKO A A, PARFENOV O G. Study of thermal decomposition of aluminum chloride in a plasma-chemical reactor [C]//15th International Conference on the Methods of Aerophysical Research – ICMAR 2010. Novosibirsk, Russia, Abstracts Part II, 2010: 155-156.
[8] ZHU Fu-long, YANG Bin, YUAN Hai-bin, YU Qing-chun, XU Bao-qiang, DAI Yong-nian. Silica behavior in the alumina carbothermic reduction-chlorination process [J]. JOM, 2011, 63(8): 116-19.
[9] KUSTOV A D, PARFENOV O G. Method of producing chlorine from hydrogen chloride using tungsten containing compounds: Russian Federation, 2485046 [P]. 2012-01-10. (in Russian)
[10] KUSTOV A D, PARFENOV O G. Method of producing sulphur from hydrogen sulphide: Russian Federation, 2448040 [P]. 2010-11-30. (in Russian)
[11] KUSTOV A D, PARFENOV O G. High speed aluminum metallurgy [J]. Doklady Chemistry, 2015, 462(2): 149-151.
[12] KUSTOV A D, PARFENOV O G, SOLOVYOV L A, VERESHCHAGIN S N. Kyanite ore processing by carbochlorination [J]. Int J of Mineral Processing, 2014, 126(10): 70-75.
[13] RIETVELD H. A profile refinement method for nuclear and magnetic structures [J]. J Appl Crystallogr, 1969, 2: 65-71.
[14] SOLOVYOV L A. Full-profile refinement by derivative difference minimization [J]. J Appl Crystallogr, 2004, 37: 743-749.
[15] SMITH K A, RIEMER S C, IWASAKI I. Carbochlorination of aluminum from non-bauxite sources [J]. JOM, 1982, 34(9): 59-62.
[16] GURVICH L V, VEYTS I V, MEDVEDEV V A, et al. Thermodynamic properties of individual substances [M]. Vol. I–III. 4th edition [M]. New York: Hemisphere Publ Corp, Begell House and CRC Press, 1989-1994.
[17] SHARMA R A. Liquidus and eutectic phase equilibria in the systems CaI2-CaCl2, CaI2-CaF2, and CaI2-MgCl2 [J]. High Temp Sci, 1969, 1: 423-429.
[18] OKAMOTO H. Al-Zn (Aluminum-Zinc) [J]. J Phase Equilibria, 1995, 16(3): 281-282.
[19] GREENWOOD N N, EARNSHAW A. The chemistry of the elements [M]. 2nd ed. Oxford: Butterworth – Heinemann, 1997.
[20] KUSTOV A D, PARFENOV O G. Decomposition method of calcium-containing mineral raw material: Russian Federation, 2440432 [P]. 2010-12-15. (in Russian)
[21] KUSTOV A D, PARFENOV O G. Method of obtaining chlorine from calcium chloride: Russian Federation, 2503487 [P]. 2012-07-30. (in Russian)
[22] HILPERT K, BENCIVENNI L, SAHA B. Thermochemistry of the molecule (ZnI2)2(g) and the vaporization of ZnI2(s) [J]. J of Chem Phys, 1985, 83: 5227-5230.
[23] HABASHI F. Handbook of extractive metallurgy [M]. Vol. 2. Weinheim: Wiley-VCH, 1997.
[24] WEI Qin-shuai, YANG Bin, LI Yi-fu, DAI Yong-nian. Zinc removing from aluminum alloy by vacuum distillation [J]. Advanced Mater Res, 2012, 402: 303-306.
[25] EGAN D R, PONCE DE LEУN C, WOOD R J K, JONES R L, STOKES K R, WALSH F C. Developments in electrode materials and electrolytes for aluminum-air batteries [J]. J of Power Sources, 2013, 236: 293-310.
[26] Mineral commodity summaries 2015 [R]. U.S. Geological Survey, 2015. DOI:10.3133/70140094.
[27] XUE Bo-fei, FU Zheng-wen, LI Hong, LIU Xi-zhe, CHENG Sun-chao, YAO Jia, et al. Cheap and environmentally benign electrochemical energy storage and conversion devices based on AlI3 electrolytes [J]. J Am Chem Soc, 2006, 128: 8720-8721.
[28] YI Xiao-bing. Brief introduction of the primary aluminium industry of China [C]//Joint Australia-China Aluminium Industry Technology Symposium 2013. Melbourne, Australia, Swinburne University of Technology, 2013: 5-13.
Oleg G. PARFENOV, Andrey D. KUSTOV, Leonid A. SOLOVYOV
Institute of Chemistry and Chemical Technology of SB RAS, Akademgorodok 50/24, Krasnoyarsk 660036, Russia
摘 要:提出了一种从氧化铝中提取原铝的新方法。该方法的原理基于如下化学反应:AlI3+(3/2)Zn=Al+(3/2)ZnI2。首先通过氧化铝的碳热氯化反应得到氯化铝(AlCl3),然后通过氯化铝和碘化钙的交换反应(AlCl3+CaI2→ AlI3+CaCl2)得到碘化铝。在实验室条件下对这些反应进行了研究,同时对一些回收主要化学试剂的反应进行了研究(Cl2, ZnI2→Zn, CaCl2→CaI2)。采用XRD和SEM对反应产物的相组成及形貌进行分析。采用总自由能最小方法对化学反应平衡进行计算。结果表明,采用非电解方法,可以在没有非常高的温度和可消耗的化学试剂的条件下,从氧化铝中有效地提取铝。与当今采用燃煤发电提供电力的铝电解厂相比,本方法所产生的单位碳消耗和CO2大气排放可以降低一半以上。
关键词:铝;铝合金;氯化物;碘化物;锌;碘;高速冶金
(Edited by Sai-qian YUAN)
Corresponding author: Andrey D. KUSTOV; Tel: +7-391-2051951; E-mail: kustov@icct.ru
DOI: 10.1016/S1003-6326(16)64373-X

