一种通用的相转换策略构建BiVO4纳米薄片@WO3阵列异质结光阳极
来源期刊:中国有色金属学报(英文版)2021年第2期
论文作者:苏昕 刘灿军 刘洋 杨亚辉 刘玄 陈述
文章页码:533 - 544
关键词:光阳极;钒酸铋;氧化钨;异质结
Key words:photoanode; bismuth vanadate; tungsten oxide; heterojunction
摘 要:提出一种制备BiVO4纳米薄片@WO3纳米棒和纳米片薄膜的相转换策略。这种策略包括三步水热过程(WO3→WO3/Bi2WO6→WO3/BiVO4)。表征结果表明:大量的BiVO4纳米薄片原位生长在WO3纳米棒和纳米片阵列薄膜表面,从而形成WO3/BiVO4异质结。制备的WO3/BiVO4异质结薄膜作为光阳极被用于光电化学分解水,并展现出优异的光电化学活性。在可见光照射和没有沉积助催化剂的情况下,WO3/BiVO4 纳米棒和纳米片光阳极的光电流密度分别达到约1.56和1.20 mA/cm2(V=1.23 V (vs RHE))。
Abstract: A versatile phase transformation strategy was proposed to synthesize novel BiVO4 nanosheets (NSs)@WO3 nanorod (NR) and nanoplate (NP) arrays films. The strategy was carried out by following a three-step hydrothermal process (WO3→WO3/Bi2WO6→WO3/BiVO4). According to the characterization results, plenty of BiVO4 NSs grew well on the surface of WO3 NR and NP arrays films, thus forming the WO3/BiVO4 heterojunction structure. The prepared WO3/BiVO4 heterojunction films were used as the photoanodes for the photoelectrochemical (PEC) water splitting. As indicated by the results, the photoanodes exhibited an excellent PEC activity. The photocurrent densities of the WO3/BiVO4 NR and NP photoanodes at 1.23 V (vs RHE) without cocatalyst under visible light illumination reached up to about 1.56 and 1.20 mA/cm2, respectively.
Trans. Nonferrous Met. Soc. China 31(2021) 533-544
Xin SU1, Can-jun LIU1, Yang LIU2, Ya-hui YANG3, Xuan LIU1, Shu CHEN1
1. Key Laboratory of Theoretical Organic Chemistry and Function Molecule of Ministry of Education, School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
3. School of Chemistry and Chemical Engineering, Hunan Normal University, Changsha 410081, China
Received 12 March 2020; accepted 21 December 2020
Abstract: A versatile phase transformation strategy was proposed to synthesize novel BiVO4 nanosheets (NSs)@WO3 nanorod (NR) and nanoplate (NP) arrays films. The strategy was carried out by following a three-step hydrothermal process (WO3→WO3/Bi2WO6→WO3/BiVO4). According to the characterization results, plenty of BiVO4 NSs grew well on the surface of WO3 NR and NP arrays films, thus forming the WO3/BiVO4 heterojunction structure. The prepared WO3/BiVO4 heterojunction films were used as the photoanodes for the photoelectrochemical (PEC) water splitting. As indicated by the results, the photoanodes exhibited an excellent PEC activity. The photocurrent densities of the WO3/BiVO4 NR and NP photoanodes at 1.23 V (vs RHE) without cocatalyst under visible light illumination reached up to about 1.56 and 1.20 mA/cm2, respectively.
Key words: photoanode; bismuth vanadate; tungsten oxide; heterojunction
1 Introduction
The renewable generation of the clean fuels plays an essential role in meeting the growing demand for the energy and reversing environmental deterioration [1,2]. Photoelectrochemical (PEC) water splitting is potentially an ideal approach to obtaining hydrogen fuels directly from sunlight [3]. Since the first report on TiO2 film photoanode in 1972 [4], plenty of efforts have been devoted to developing a high-efficiency photoanode for PEC water splitting over the past decades [5]. Up to now, some semiconductor materials have been applied as photoanodes, such as ZnO [6], Fe2O3 [7], WO3 [8], TiO2 [9] and BiVO4 [10].
Especially, WO3 is widely recognized as a promising photoanode material for its nontoxicity, high electrochemical stability and excellent charge- transferring ability [11]. However, the solar-to- hydrogen efficiency (STHE) of single WO3 photoanode is low due to its poor ability to absorb visible light and the high recombination rate of photogenerated carriers [12]. Thus, some strategies have been proposed to address these issues, including morphology control [13], element doping [14], and heterojunction constructing [15], etc. Particularly, the construction of heterojunction with other narrow-gap semiconductors has been confirmed as an effective method in promoting the spatial separation of photogenerated carriers and extending the visible-light response simultaneously.
As a narrow-gap semiconductor, BiVO4 is considered to be one of the most promising materials fit for the construction of type-II alignment heterojunction with WO3, due to its excellent performance in absorbing visible light (λ≤520 nm), high chemical stability and desirable conduction band (CB) position [16,17]. Therefore, WO3/BiVO4 films as photoanodes have been extensively studied [18]. Applying a solvothermal technique, SU et al [19] prepared a novel WO3/BiVO4 nanorod (NR) arrays photoanode that achieved a higher PEC activity than the planar WO3/BiVO4 photoanode. SHI et al [20] constructed a WO3/BiVO4 helix nanostructure heterojunction that demonstrated such advantages as effective light scattering, large contact surface area as well as high rate of carriers separation and transportation. Besides, its performance was verified through theoretical simulation and analysis. LEE et al [21] prepared a 1D WO3/BiVO4 photoanode by depositing dot-like BiVO4 on the WO3 NR surface. A systematic analysis was conducted to reveal the vital role played by the optimization of WO3 NR morphology in achieving high PEC efficiency. According to the aforementioned studies, the morphology and nanostructure of WO3/BiVO4 heterojunction play a significant role in improving the PEC efficiency.
Recently, an in-situ transformation method has been widely applied to constructing heterostructure. Taking a anion transformation approach, GAO et al [22] prepared novel BiVO4/Bi2S3 hollow nano- discoidals that exhibited superior photocatalytic activity. CHITRADA et al [23] produced Bi2O3- BiO2-x photoanode using the in-situ photo- conversion method and the formation of photo- converted Bi2O4-x phase was conducive to absorbing more visible light, thus leading to high photocurrent density. The in-situ transformation method is considered to be more effortless to adjust the morphology and nanostructure of heterostructure compared with other methods. Besides, the heterostructure constructed using this method shows a reduced interface defect [24]. Thus, it is necessary to develop an in-situ transformation to prepare WO3/BiVO4 hetero- junctions with various morphologies and study their PEC performance.
In this study, a versatile phase transformation strategy was applied to designing and fabricating novel BiVO4 NS@WO3 NR and NP arrays photo- anodes. The strategy was performed via a three- step hydrothermal process (WO3→WO3/Bi2WO6→ WO3/BiVO4). The morphology and nanostructure of samples prepared by the strategy were characterized and the corresponding PEC properties were investigated.
2 Experimental
2.1 Synthesis of WO3 NR and NP arrays on FTO substrate
WO3 NR arrays were prepared using a hydrothermal method as described in the previous reports [25]. Firstly, 0.15 g of ammonium paratungstate was dissolved in 15 mL of deionized water. Then, 0.6 mL of 12 mol/L HCl and 0.3 mL of H2O2 were added in sequence into the above- mentioned aqueous solution by stirring. Then, an FTO substrate was immersed in 20 mL of Teflon- lined autoclave and placed against the wall with the conductive side downward. Subsequently, the aforementioned precursor solution was poured into the autoclave and the hydrothermal process was conducted at 170 °C for 4 h. Finally, the obtained films were annealed at 300 °C for 1 h before further use.
WO3 NP arrays were synthesized using a hydrothermal method as described in the previous reports [26]. Firstly, 0.25 g of Na2WO4·2H2O was added into 30 mL of deionized water by stirring. Then, 6 mL of 3 mol/L HCl, 30 mL of deionized water and 0.20 g of (NH4)2C2O4 were added into the aforementioned solution and stirred for 30 min. Then, an FTO substrate was immersed in 100 mL of Teflon-lined autoclave and placed against the wall with the conductive side downward. Afterwards, the above-mentioned precursor solution was poured into the autoclave and the hydrothermal process was conducted at 140 °C for 6 h. Finally, the obtained films were annealed at 300 °C for 1 h before further use.
2.2 Synthesis of WO3/Bi2WO6 NR and NP arrays on FTO substrate
The WO3/Bi2WO6 films were prepared through hydrothermal treatment. Firstly, the hydrothermal reaction solution was obtained by adding 0.6 g of Bi(NO3)3·5H2O as the Bi source into 30 mL of 0.2 mol/L HNO3 solution. Then, the WO3 NR or WO3 NP arrays films were immersed in 50 mL of Teflon-lined autoclave and placed against the wall with the sample side facing down. Then, the aforementioned precursor solution was poured into the autoclave and the hydrothermal process was conducted at 200 °C for 10 h. Finally, the obtained films were annealed at 540 °C for 4 h before further use.
2.3 Synthesis of WO3/BiVO4 NR and NP arrays on FTO substrate
The WO3/BiVO4 films were prepared through hydrothermal treatment. Firstly, the precursor solution was prepared by adding 4 mg of NH4VO3 and 0.2 mL of 3 mol/L HCl into 30 mL of deionized water. Then, the WO3/Bi2WO6 films were immersed in 50 mL of Teflon-lined autoclave and placed against the wall with the sample side facing down. Then, the above-mentioned solution was transferred into the autoclave and placed in an oven at 180 °C for 4 h. Subsequent to the reaction, the formed films were calcined at 500 °C for 1 h before further use.
2.4 Characterization
The crystal, morphological and optical characteristics were examined using X-ray diffractometer (XRD, D8 Advance, AXS), transmission electron microscope (TEM, Titan G2 60-300, FEI), field emission scanning electron microscope (FE-SEM, MIRA3, TESCAN) and UV-vis spectrophotometer (UV-vis, 2450, Shimadzu).
2.5 PEC measurement
The PEC measurements were performed in a three-electrode PEC cell on an electrochemical workstation (Zahner, Zennium, Germany). 0.5 mol/L of KH2PO4 (pH≈4.1) was treated as the electrolyte. A 500 W Xe lamp (CHF-XM, Perfectlight) coupled with a 400 nm cutoff filter was applied as the light source and the light intensity at the photoanode position was adjusted to 100 mW/cm2, as measured using a light power meter (PL-MW2000, Perfectlight). The electro- chemical impedance spectra (EIS) were recorded at 1.23 V (vs RHE) with a frequency of 104-0.1 Hz.
3 Results and discussion
3.1 Microstructure
WO3/BiVO4 arrays heterojunction films were fabricated using a three-step hydrothermal strategy and the fabrication process is illustrated in Fig. 1. The WO3 NR and NP arrays films were initially prepared according to the previous reports [25,26]. Subsequently, the Bi2WO6 NS developed on the surface of WO3 NR and NP arrays films under hydrothermal conditions as a result of the chemical reaction is shown as
2Bi3++WO3+3H2O→Bi2WO6+6H+ (1)
Finally, Bi2WO6 NS was transformed into BiVO4 NS on the surface of WO3 to obtain BiVO4 NS@WO3 arrays films. The potential reaction is expressed as
 →
→ (2)
(2)
The microstructure and morphology of the samples were characterized using SEM and TEM. According to the SEM images of the WO3 NR (Figs. 2(a, b)) and NP (Figs. 2(g, h)) arrays films, both WO3 NR and NP had vertically grown on the FTO substrate after the hydrothermal treatment and their surface was smooth. As shown in the cross-sectional view images, the length of WO3 NR is approximately 2.5 μm and WO3 NP ranges from 0.5 to 1 μm in edge length and from 80 to 200 nm in thickness. After the second-step hydrothermal treatment, the surface of WO3 NR and NP was rough and plenty of Bi2WO6 NS has epitaxially grown on the WO3 surface, thus leading to the generation of WO3/Bi2WO6 NR (Figs. 2(c, d)) and NP (Figs. 2(i, j)) films, which is attributed to the in-situ formation of Bi2WO6 NS as a result of the reaction occurring between WO3 and Bi3+ under hydrothermal conditions. As shown in Figs. 2(e, f) and Figs. 2(k, l), there are some small NSs adhering on the surface of WO3 NR and NP, because the Bi2WO6 NS on the WO3 surface could be transformed into BiVO4 NS by the third-step hydrothermal treatment, indicating the formation of WO3/BiVO4 NR and NP heterojunction films.

Fig. 1 Schematic illustration of preparation process for WO3/BiVO4 NR and NP arrays films

Fig. 2 SEM images of WO3 NR (a, b), WO3/Bi2WO6 NR (c, d), WO3/BiVO4 NR (e, f), WO3 NP (g, h), WO3/Bi2WO6 NP (i, j) and WO3/BiVO4 NP (k, l) arrays films
According to the TEM images of WO3/BiVO4 NR (Figs. 3(a-c)) and NP (Figs. 3(d-f)), WO3 NR has a rod-like nanostructure and WO3 NP possesses a plate-like nanostructure, which is consistent with the SEM images. As shown in LRTEM image, the surfaces of WO3 NR and NP are covered with a large number of NSs. According to the HRTEM images, NS exhibits clear lattice fringes with spacings of 0.260 and 0.254 nm, which are consistent with the (200) and (020) crystal planes of monoclinic BiVO4, respectively. By contrast, the NP shows a lattice spacing of 0.335 nm, corresponding to the (120) crystal plane of the monoclinic WO3.
Furthermore, as suggested by the elemental mappings of WO3/BiVO4 NR (Fig. 4(a)) and NP (Fig. 4(b)), W, V and Bi elements are distributed uniformly, indicating the formation of BiVO4 NS@WO3 arrays heterojunction films.

Fig. 3 TEM images of WO3/BiVO4 NR (a-c) and NP (d-f)

Fig. 4 Elemental mapping images of WO3/BiVO4 NR (a) and NP (b)

Fig. 5 XRD patterns (a, b) and UV-vis absorption spectra (c, d) of prepared film
The phase and crystallinity of WO3, WO3/Bi2WO6 and WO3/BiVO4 films were characterized by XRD, as shown in Figs. 5(a, b). The obtained WO3 arrays films exhibit the characteristic diffraction peaks of monoclinic WO3 (JCPDS 83-0950). As for the WO3/Bi2WO6 films, the observed peaks of characteristic diffraction at 28.31° and 32.93° are assigned to the (113) and (020) crystal planes of orthorhombic Bi2WO6 (JCPDS 73-2020), respectively. Corresponding to the (011), (112) and (004) crystal planes of monoclinic BiVO4, new peaks observed at 18.98°, 28.95° and 30.53° are distinguishable from the XRD patterns of WO3/BiVO4 films (JCPDS 75-2480), suggesting the formation of BiVO4 on the surface of WO3 films. Figures 5 shows the spectra of UV-vis absorption. The optical absorption of WO3 films shows similarity to WO3/Bi2WO6 films, with the edges of their absorption being around 470 nm, which corresponds to their bandgap energy (Eg≈2.7 eV). In addition, WO3/BiVO4 films show a visible redshift and their absorption edges are about 520 nm, which is consistent with the bandgap energy of BiVO4 (Eg≈2.4 eV). As a result, the absorption of visible light by the films can be improved significantly by the formation of BiVO4 on the surface of WO3. As shown in Figs. 5(c, d), the images of the prepared films also are consistent with the UV-vis absorption results. WO3 NR and NP films are yellow-green while WO3/BiVO4 films are bright yellow.
3.2 PEC performance of WO3/BiVO4 photo- anodes
In order to assess the PEC performance, the chopped linear sweep voltammetry (LSV) plots were obtained in 0.5 mol/L KH2PO4 electrolyte under visible light. As shown in Figs. 6(a, b), the photocurrent density of the WO3/BiVO4 NR and NP photoanodes is significantly improved after the formation of BiVO4 on the surface of WO3 arrays. The photocurrent densities of WO3/BiVO4 NR and NP photoanodes reach ca. 1.56 and 1.21 mA/cm2 at 1.23 V (vs RHE), respectively, which are much higher compared with pure WO3 NR and NP photoanodes. More importantly, the photocurrent density and onset potential of the prepared WO3/BiVO4 photoanodes are comparable to the reported WO3/BiVO4 photoanodes without cocatalysts, as indicated in Table 1.
In this study, the incident photon-to-electron conversion efficiency (IPCE) was examined to figure out the relationship between photocurrent density and light absorption. The IPCE plots were recorded at a constant potential of 1.23 V(vs RHE), as shown in Figs. 6(c, d). It can be seen that WO3/BiVO4 NR and NP photoanodes achieve the highest IPCE value in the overall wavelength range due to the type-II heterojunction structure of the WO3/BiVO4 photoanodes, which is conducive to the separation and transfer of photogenerated charge carriers. Furthermore, WO3/BiVO4 photo- anodes show an extended photo-responsive range (~520 nm) due to the narrower bandgap energy of BiVO4.
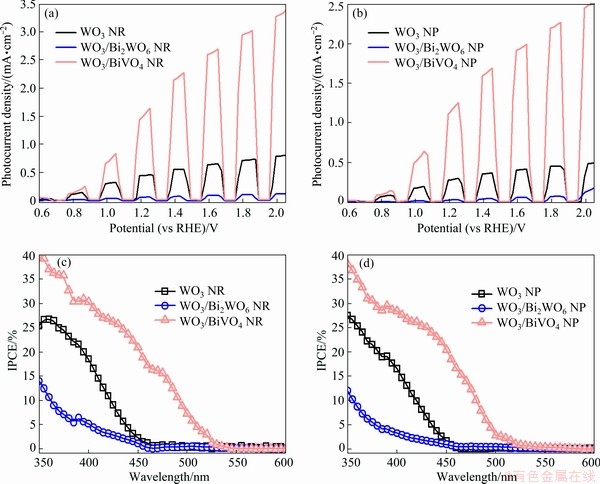
Fig. 6 LSV plots (a, b) and IPCE spectra (c, d) of as-prepared photoanode
Table 1 PEC performance of WO3/BiVO4 photoanode without cocatalyst in previous literatures

To evaluate the ability of charge transfer, the EIS of the photoanodes was measured within the frequency range of 104-10-1 Hz at 1.23 V (vs RHE) under visible light, as shown in Fig. 7. All photoanodes show a single semicircle, suggesting that the charge transfer across the photoelectrode/ electrolyte interface is the only rate-limiting step. Compared with the WO3 and WO3/Bi2WO6 photoanodes, WO3/BiVO4 photoanodes exhibit a smaller arc radius, indicating the better performance achieved by WO3/BiVO4 photoanode in interfacial charge transfer.
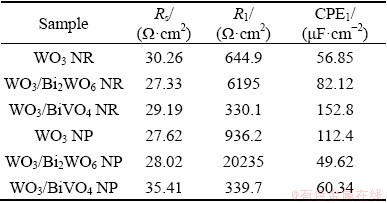
In addition, the EIS data are fitted by the equivalent circuit consisting of a solution resistance (Rs), a charge transfer resistance (R1) and a constant phase element (CPE1), as shown in the inset in Fig. 7. As indicated by the fitted results listed in Table 2, WO3/BiVO4 photoanodes have the smallest R1 value, indicating the lowest resistance to charge transfer at the interface between WO3/BiVO4 photoanode and electrolyte solution. The EIS results conform to the LSV plots.

Fig. 7 EIS spectra of as-prepared NR (a) and NP (b) arrays photoanodes
Table 2 Fitted values of equivalent circuit in PEC water splitting
Besides, the efficiency of WO3/BiVO4 photoanodes in charge transfer was estimated according to ηtrans=JH2O/JNa2SO3, as shown in Fig. 8. The ηtrans values at 1.23 V (vs RHE) of WO3/BiVO4 NR and NP photoanodes are 65.6% and 74.9%, respectively. It is speculated that the low values of ηtrans result from the absence of cocatalyst on the surface of photoanode.
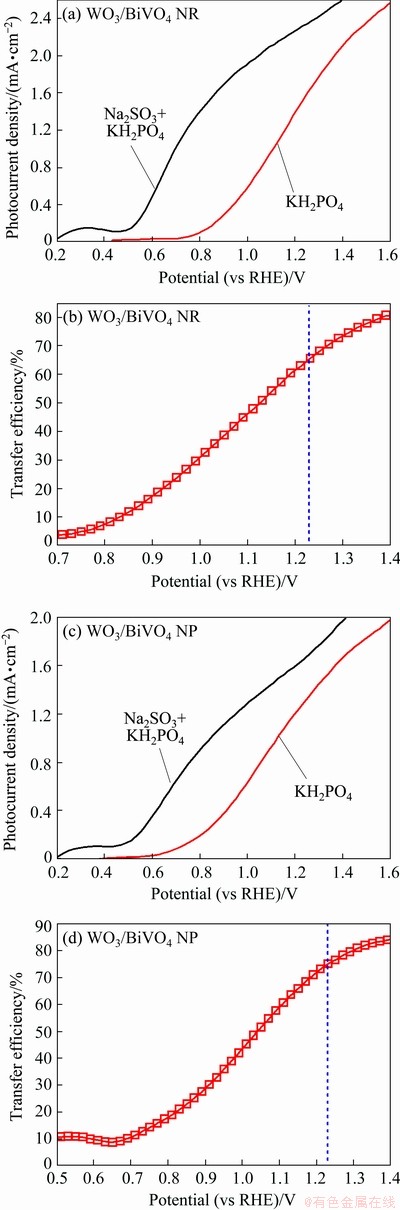
Fig. 8 Photocurrent density curves (a, c) in 0.5 mol/L Na2SO3 and KH2PO4 solution and surface charge transfer efficiency (b, d) of BiVO4/WO3 NR (a, b) and NP (c, d) arrays photoanodes
The PEC stability of WO3/BiVO4 photoanodes was evaluated at 1.23 V (vs RHE) under long-term irradiation, as shown in Fig. 9. The photocurrent density of WO3/BiVO4 photoanodes is stable and maintained about 90% after 2 h, indicating their excellent PEC stability in the water-splitting process.
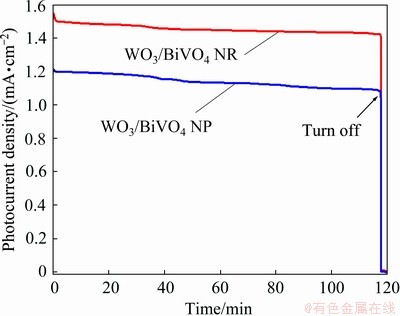
Fig. 9 Photocurrent density-time curve of WO3/BiVO4 NR and NP arrays photoanodes
In order to confirm the flat band potentials (Vfb) of the prepared photoanodes, Mott-Schottky (MS) plots were measured in darkness in a 0.5 mol/L KH2PO4 solution with applied frequencies of 1, 2.5 and 5 kHz, respectively. The Vfb and Nd could be estimated using the MS equation [33]:
 (3)
(3)
where C represents the specific capacitance, ε0 indicates the permittivity of vacuum, ε denotes the dielectric constant of the electrode material used, q is the electron charge, VE stands for the applied potential, Nd means the donor density, and k is the Boltzmann constant. As shown in Fig. 10, all M-S plots exhibit a positive slope, implying that the photoanode materials are n-type semiconductors. Vfb can be obtained from the intercept of the fitted tangent line. Compared with the WO3 photoanode, WO3/BiVO4 photoanodes show a more negative Vfb, suggesting the possibility that the photogenerated electrons are transferred from BiVO4 to WO3 due to the more negative CB edge of BiVO4.
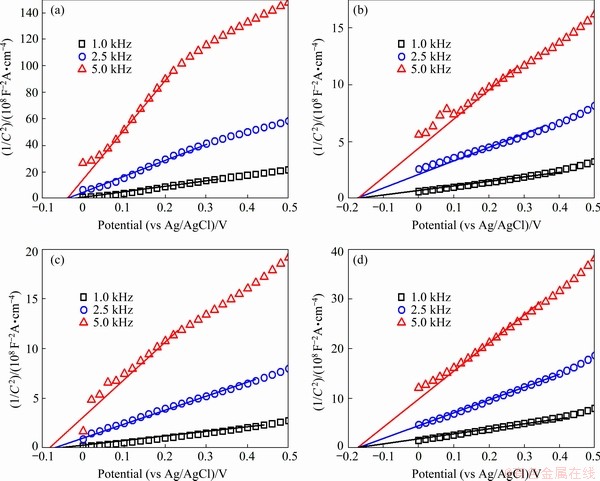
Fig. 10 MS plots of WO3 NR (a), WO3/BiVO4 NR (b), WO3 NP (c) and WO3/BiVO4 NP (d) arrays photoanodes
3.3 Mechanism of charge separation and transfer of WO3/BiVO4 photoanode
Figure 11 illustrates the mechanism of charge separation and transfer. With the phase transformation strategy applied, the dense BiVO4 NS was coated on the WO3 surface, thus forming a WO3/BiVO4 type-II heterojunction. Under irradiation, the electrons in BiVO4 and WO3 were excited from their valence band (VB) to the CB. Due to the more negative CB position of BiVO4, the excited electrons in the CB of BiVO4 were easily transferred to the CB of WO3. These electrons were further moved onto the FTO substrate along the WO3 NR and NP pathway and then to the Pt electrode through the external circuit. Then, the H+ in the electrolyte reacted with the electrons to generate H2 on the surface of Pt electrode. In the meantime, the photoexcited holes shifted from WO3 to the VB of BiVO4 and oxidized H2O to produce oxygen on the BiVO4 surface. Thus, type-II heterojunction can significantly improve the outcome of charge separation and transfer.
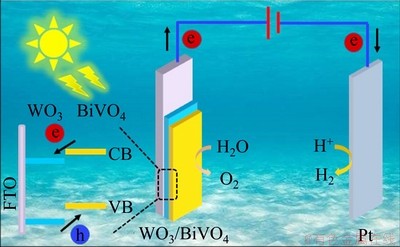
Fig. 11 Schematic illustration for charge separation and transfer of WO3/BiVO4 photoanode
4 Conclusions
(1) A versatile strategy of phase transformation was proposed to prepare BiVO4 NS@WO3 NR and NP arrays films. The strategy was carried out via a three-step hydrothermal process (WO3→WO3/ Bi2WO6→WO3/BiVO4).
(2) As indicated by the SEM, TEM and XRD results, plenty of BiVO4 NS successfully formed on the surface of the WO3 NR and NP arrays films with Bi2WO6 NS as the intermediate product.
(3) The prepared WO3/BiVO4 photoanodes were applied for PEC water splitting and exhibited a significantly higher PEC activity compared with WO3 photoanodes. It is believed that the proposed strategy is promising in the construction of various heterojunction systems for the future.
Acknowledgments
The authors are grateful for the financial supports from the National Natural Science Foundation of China (21808051, 51904356, 21703062).
References
[1] Turner J A. Sustainable hydrogen production[J]. Science, 2004, 305(5686): 972-974.
[2] WAN Xiao-kang, WANG Lu, DONG Chung-li, MENENDEZ R G, HUANG Yu-cheng, MACCHIONI A, SHEN Shao-hua. Activating klaui-type organometallic precursors at metal oxide surfaces for enhanced solar water oxidation[J]. ACS Energy Letters, 2018, 3(7): 1613-1619.
[3] Wang S, Liu G, Wang L. Crystal facet engineering of photoelectrodes for photoelectrochemical water splitting[J]. Chemical Reviews, 2019, 119(8): 5192-5247.
[4] Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode [J]. Nature, 1972, 238(5358): 37-38.
[5] CAO Guo-jian, CUI Bo, WANG Wen-qi, TANG Guang-ze, FENG Yi-cheng, WANG Li-ping. Fabrication and photodegradation properties of TiO2 nanotubes on porous Ti by anodization [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(8): 2581-2587.
[6] CAO Chang, XIE Xin-xin, ZENG Ya-mei, SHI Shao-hua, WANG Gui-zhen, YANG Liang, WANG Cai-zhuang, LIN Shi-wei. Highly efficient and stable p-type ZnO nanowires with piezotronic effect for photoelectrochemical water splitting [J]. Nano Energy, 2019, 61: 550-558.
[7] MAO Lian-lian, HUANG Yu-cheng, FU Yan-ming, DONG Chung-li, SHEN Shao-hua. Surface sulfurization activating hematite nanorods for efficient photoelectrochemical water splitting [J]. Science Bulletin, 2019, 64(17): 1262-1271.
[8] SOLTANI T, TAYYEBI A, HONG H, MIRFASIH M H, LEE B K. A novel growth control of nanoplates WO3 photoanodes with dual oxygen and tungsten vacancies for efficient photoelectrochemical water splitting performance [J]. Solar Energy Materials and Solar Cells, 2019, 191: 39-49.
[9] Yang Ya-hui, Xie Ren-rui, Li Hang, Liu Can-jun, Liu Wen-hua, Zhan Fa-qi. Photoelectrocatalytic reduction of CO2 into formic acid using WO3-x/TiO2 film as novel photoanode [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(9): 2390-2396.
[10] Chen Yu-shiang, Lin Lu-yin. Novel synthesis of highly ordered BiVO4 nanorod array for photoelectrochemical water oxidation using a facile solution process [J]. Journal of Power Sources, 2019, 436: 226842.
[11] Chandra D, Saito K, Yui T, Yagi M. Tunable mesoporous structure of crystalline WO3 photoanode toward efficient visible-light-driven water oxidation [J]. ACS Sustainable Chemistry & Engineering, 2018, 6(12): 16838-16846.
[12] Zhu T, Chong M N, Chan E S. Nanostructured tungsten trioxide thin films synthesized for photoelectrocatalytic water oxidation: A review [J]. ChemSusChem, 2014, 7(11): 2974-2997.
[13] APOLINARIO A, LOPES T, COSTA C, ARAUJO J P, MENDES A M. Multilayered WO3 nanoplatelets for efficient photoelectrochemical water splitting: The role of the annealing ramp [J]. ACS Applied Energy Materials, 2019, 2(2): 1040-1050.
[14] Liu Yang, Li Jie, Li Wen-zhang, Yang Ya-hui, Li Yao-min, Chen Qi-yuan. Enhancement of the photoelectrochemical performance of WO3 vertical arrays film for solar water splitting by gadolinium doping [J]. The Journal of Physical Chemistry C, 2015, 119(27): 14834-14842.
[15] BAI Shou-li, YANG Xiao-jun, LIU Cheng-yao, XIANG Xu, LUO Rui-xian, HE Jing, CHEN Ai-fan. An integrating photoanode of WO3/Fe2O3 heterojunction decorated with NiFe-LDH to improve PEC water splitting efficiency [J]. ACS Sustainable Chemistry & Engineering, 2018, 6(10): 12906-12913.
[16] Li Hai-bin, Zhang Jian, Huang Guo-you, Fu Sheng-hao, Ma Chao, Wang Bai-yu, Huang Qian-ru, Liao Hong- wei. Hydrothermal synthesis and enhanced photocatalytic activity of hierarchical flower-like Fe-doped BiVO4 [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(4): 868-875.
[17] WANG Min, YANG Guang-jun, YOU Mei-yan, XIE Yuan-hua, WANG You-zhao, HAN Jin, ZHU Tong. Effects of Ni doping contents on photocatalytic activity of B-BiVO4 synthesized through sol-gel and impregnation two-step method [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(9): 2022-2030.
[18] Grigioni I, Abdellah M, Corti A, Dozzi M. V, Hammarstrom L, Selli E. Photoinduced charge- transfer dynamics in WO3/BiVO4 photoanodes probed through mid-IR transient absorption spectroscopy [J]. Journal of the American Chemical Society, 2018, 140(43): 14042-14045.
[19] Su J, Guo L, Bao N, Grimes C A. Nanostructured WO3/BiVO4 heterojunction films for efficient photoelectrochemical water splitting [J]. Nano Letter, 2011, 11(5): 1928-1933.
[20] Shi X, Choi I Y, Zhang K, Kwon J, Kim D Y, Lee J K, Oh S H, Kim J K, Park J H. Efficient photoelectrochemical hydrogen production from bismuth vanadate-decorated tungsten trioxide helix nanostructures [J]. Nature communications, 2014, 5: 4775.
[21] Lee M G, Kim D H Sohn W, Moon C W, Park H, Lee S, Jang H W. Conformally coated BiVO4 nanodots on porosity-controlled WO3 nanorods as highly efficient type II heterojunction photoanodes for water oxidation [J]. Nano Energy, 2016, 28: 250-260.
[22] GAO Xue-hui, WU Hao-bin, ZHENG Ling-xia, ZHONG Yi-jun, HU Yong, LOU Xiong-wen. Formation of mesoporous heterostructured BiVO4/Bi2S3 hollow discoids with enhanced photoactivity [J]. Angewandte Chemie, 2014, 126(23): 6027-6031.
[23] CHITRADA K C, GAKHAR R, CHIDAMBARAM D, ASTON E, RAJA K S. Enhanced performance of β-Bi2O3 by in-situ photo-conversion to Bi2O3-BiO2-x composite photoanode for solar water splitting [J]. Journal of the Electrochemical Society, 2016, 163(7): H546-H558.
[24] Liu Can-jun, Yang Ya-hui, Li Wen-zhang, Li Jie, Li Yao-min, CHEN Qi-yuan. In situ synthesis of Bi2S3 sensitized WO3 nanoplate arrays with less interfacial defects and enhanced photoelectrochemical performance [J]. Scientific Reports, 2016, 6: 23451.
[25] LIU Yang, WYGANT B R, KAWASHIMA K, MABAYOJE O, HONG T E, LEE S G, LIN Jie, KIM J H, YUBUTA K, LI Wen-zhang, LI Jie, MULLINS C B. Facet effect on the photoelectrochemical performance of a WO3/BiVO4 heterojunction photoanode [J]. Applied Catalysis B: Environmental, 2019, 245: 227-239.
[26] Yang Jiao, Li Wen-zhang, Li Jie, Sun Di-bo, CHEN Qi- yuan. Hydrothermal synthesis and photoelectrochemical properties of vertically aligned tungsten trioxide (hydrate) plate-like arrays fabricated directly on FTO substrates [J]. Journal of Materials Chemistry, 2012, 22(34): 17744-17752.
[27] Hong S J, Lee S, Jang J S, Lee J S. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation [J]. Energy & Environmental Science, 2011, 4(5): 1781-1787.
[28] Xia Li-gang, Bai Jing, Li Jin-hua, Zeng Qing-yi, Li Xue-jin, Zhou Bao-xue. A highly efficient BiVO4/WO3/W heterojunction photoanode for visible-light responsive dual photoelectrode photocatalytic fuel cell [J]. Applied Catalysis B: Environmental, 2016, 183: 224-230.
[29] GRIGIONI I, CORTI A, DOZZI M V, SELLI E. Photoactivity and stability of WO3/BiVO4 photoanodes: effects of the contact electrolyte and of Ni/Fe oxyhydroxideprotection [J]. The Journal of Physical Chemistry C, 2018, 122(25): 13969-13978.
[30] Ma Zi-zai, Hou Hui-lin, Song Kai, Fang Zhi, Wang Lin, Gao Feng-mei, Yang Zuo-bao, Tang Bin, Yang Wei-you. Ternary WO3/Porous-BiVO4/FeOOH hierarchical architectures: Towards highly efficient photoelectrochemical performance [J]. ChemElectroChem, 2018, 5(23): 3660-3667.
[31] MA Zi-zai, SONG Kai, WANG Lin, GAO Feng-mei, TANG Bin, HOU Hui-lin, YANG Wei-you. WO3/BiVO4 type-II heterojunction arrays decorated with oxygen-deficient ZnO passivation layer: A highly efficient and stable photoanode [J]. ACS Applied Materials & Interfaces, 2018, 11(1): 889-897.
[32] Xu Shang, Fu Ding-fa, Song Kai, Wang Lin, Yang Zuo-bao, Yang Wei-you, Hou Hui-lin. One-dimensional WO3/BiVO4 heterojunction photoanodes for efficient photoelectrochemical water splitting [J]. Chemical Engineering Journal, 2018, 349: 368-375.
[33] LIU Can-jun, YANG Ya-hui, LI Jie, CHEN Shu. Phase transformation synthesis of TiO2/CdS heterojunction film with high visible-light photoelectrochemical activity [J]. Nanotechnology, 2018, 29(26): 265401.
苏 昕1,刘灿军1,刘 洋2,杨亚辉3,刘 玄1,陈 述1
1. 湖南科技大学 化学化工学院 理论有机化学与功能分子教育部重点实验室,湘潭 411201;
2. 中南大学 化学化工学院,长沙 410083;
3. 湖南师范大学 化学化工学院,长沙 410081
摘 要:提出一种制备BiVO4纳米薄片@WO3纳米棒和纳米片薄膜的相转换策略。这种策略包括三步水热过程(WO3→WO3/Bi2WO6→WO3/BiVO4)。表征结果表明:大量的BiVO4纳米薄片原位生长在WO3纳米棒和纳米片阵列薄膜表面,从而形成WO3/BiVO4异质结。制备的WO3/BiVO4异质结薄膜作为光阳极被用于光电化学分解水,并展现出优异的光电化学活性。在可见光照射和没有沉积助催化剂的情况下,WO3/BiVO4 纳米棒和纳米片光阳极的光电流密度分别达到约1.56和1.20 mA/cm2(V=1.23 V (vs RHE))。
关键词:光阳极;钒酸铋;氧化钨;异质结
(Edited by Xiang-qun LI)
Corresponding author: Can-jun LIU, Tel: +86-15073161782, E-mail: liucanjun@hnust.edu.cn; Shu CHEN, E-mail: chenshu@hnust.edu.cn
DOI: 10.1016/S1003-6326(21)65515-2
1003-6326/ 2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press
2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press

