7A60铝合金点蚀行为的电化学噪声和电化学阻抗谱表征
来源期刊:中国有色金属学报(英文版)2014年第12期
论文作者:王学慧 王吉会 付丛伟
文章页码:3907 - 3916
关键词:7A60铝合金;金属间化合物;点蚀;电化学阻抗谱;电化学噪声;小波分形维数
Key words:7A60 aluminum alloy; constituent particles; pitting corrosion; electrochemical impedance spectroscopy; electrochemical noise; wavelet fractal dimension
摘 要:采用电化学阻抗谱(EIS)和电化学噪声(EN)方法研究回归再时效(RRA)热处理状态下7A60铝合金的点蚀行为,通过扫描电子显微镜(SEM)和能谱仪(EDS)观察和分析合金的组织和第二相颗粒成分。结果表明,7A60铝合金在3.5% NaCl溶液中存在两个腐蚀阶段,并且可以用EIS出现两个电容时间常数的时间和由EN计算出的小波分形维数D的变化来表征。SEM和EDS分析结果表明,在7A60铝合金中,严重的点蚀主要是由阳极相MgZn2引起的,其次是Al2MgCu和 Mg2Si相,Al7Cu2Fe 相对7A60铝合金点蚀行为的影响不大。
Abstract: The pitting corrosion behaviors of 7A60 aluminum alloy in the retrogression and re-aging (RRA) temper were investigated by electrochemical impedance spectroscopy (EIS) and electrochemical noise (EN) techniques, and the microstructure and the second phase content of the alloy were observed and determined by scanning electron microscopy (SEM) and energy dispersive spectrometer (EDS). The results show that there exist two different corrosion stages for 7A60 alloy in 3.5% NaCl solution, and the corrosion process can be detected by the appearance of EIS spectrum with two capacitive time constants and the wavelet fractal dimension D extracted from EN. SEM and EDS results also demonstrate that severe pitting corrosion in 7A60 alloy is mainly caused by electrochemical active MgZn2 particles, secondly by Al2MgCu and Mg2Si. Al7Cu2Fe particles make little contribution to the pitting corrosion of 7A60 alloy.
Trans. Nonferrous Met. Soc. China 24(2014) 3907-3916
Xue-hui WANG1,2, Ji-hui WANG1,2, Cong-wei FU2
1. State Key Laboratory of Hydraulic Engineering Simulation and Safety, Tianjin University, Tianjin 300072, China;
2. Tianjin Key Laboratory of Composite and Functional Materials, School of Materials Science and Engineering, Tianjin University, Tianjin 300072, China
Received 17 October 2013; accepted 17 November 2014
Abstract: The pitting corrosion behaviors of 7A60 aluminum alloy in the retrogression and re-aging (RRA) temper were investigated by electrochemical impedance spectroscopy (EIS) and electrochemical noise (EN) techniques, and the microstructure and the second phase content of the alloy were observed and determined by scanning electron microscopy (SEM) and energy dispersive spectrometer (EDS). The results show that there exist two different corrosion stages for 7A60 alloy in 3.5% NaCl solution, and the corrosion process can be detected by the appearance of EIS spectrum with two capacitive time constants and the wavelet fractal dimension D extracted from EN. SEM and EDS results also demonstrate that severe pitting corrosion in 7A60 alloy is mainly caused by electrochemical active MgZn2 particles, secondly by Al2MgCu and Mg2Si. Al7Cu2Fe particles make little contribution to the pitting corrosion of 7A60 alloy.
Key words: 7A60 aluminum alloy; constituent particles; pitting corrosion; electrochemical impedance spectroscopy; electrochemical noise; wavelet fractal dimension
1 Introduction
7xxx series aluminum alloys are widely used in military and aerospace industries for their low density and favorable mechanical properties [1]. However, these alloys are often suffered from pitting corrosion and stress corrosion cracking (SCC) when subjected to aggressive environments such as salt water spray and/or salt fog [2,3]. It has been reported that a superior balance of SCC resistance and strength can be obtained by retrogression and re-aging (RRA) treatment [4-6]. And our previous study has also proved that the SCC resistance of 7A60 aluminum alloy in 3.5% NaCl solution is improved by RRA treatment [7].
Al-Zn-Mg-Cu alloys contain numerous constituent particles with electrochemical potentials different from those of the matrix, then corrosion pits can readily develop in these particles [8,9]. The initiation of SCC cracks can be basically associated with intensive localized corrosion around constituent particles [10,11]. The electrochemical characteristics of intermetallic phases in Al-Zn-Mg-Cu series alloys have been studied by BIRBILIS and BUCHHEIT [12]. It is concluded that Al7Cu2Fe and Al2Cu phases are noble particles while MgZn2 phase is active particle with high self-dissolution rates. Nevertheless, the dealloying and incongruent dissolution of Al2CuMg particles may lead to polarity reversal. The pitting behaviors of 7150 and 7075 alloys under solution heat treatment and laser surface treatment have been discussed [9,10]. It is demonstrated that the pitting resistance of 7150 alloy can be improved due to the removal of active constituent particles such as η(MgZn2) and S(Al2MgCu) phases.
7A60 alloy is a new developed ultra-high strength aluminum alloy, and its SCC behaviors in RRA tempers have been studied [7]. In this work, the pitting corrosion behavior of 7A60 alloy in the RRA temper was investigated by using electrochemical impedance spectroscopy (EIS) and electrochemical noise (EN) methods, and their corroded micrographs were observed and determined by scanning electron microscopy (SEM) and energy dispersive spectrometer (EDS).
2 Experimental
2.1 Material and heat treatment
7A60 alloy used was composed of 8.0%-9.0% Zn, 2.3%-3.0% Mg, 2.0%-2.6% Cu, 0.1%-0.2% Zr, 0.09% Fe, 0.06% Si, 0.002% Be and balanced Al. The alloy was prepared by conventional casting, hot rolling and annealing at 470 °C for 2 h. And then, the hot rolled plates with a thickness of 2 mm were RRA-treated in the following sequences: 1) pre-aged at 120 °C for 24 h; 2) retrogressed at 195 °C for 180 min and 3) re-aged at 120 °C for 24 h.
2.2 Immersion test
7A60 specimens were mounted in epoxy resin with an exposed area of 2 cm2. Then, they were abraded with silicon carbide paper (from 400 to 2000 grade) and polished. The immersion test was carried out in 3.5% NaCl solution for 72 h at room temperature to evaluate the pitting corrosion behavior. The microstructure and corroded surface were observed by a TDCLS4800 scanning electron microscope, and the chemical compositions of the constituent particles were analyzed by energy dispersive spectrometer.
2.3 EIS measurements
EIS measurements of 7A60 alloy in 3.5% NaCl solution at different immersion time were carried out by using a VersaSTAT 4 electrochemical workstation with three-electrode system at open circuit potential with a 10 mV sine perturbation to the cell. The working electrode was 7A60 alloy with an exposed area of 2 cm2. The reference electrode was a saturated calomel electrode (SCE) and the counter electrode was a platinum plat. The measuring frequency range was 105-10-2 Hz. The experimental data of the impedance were analyzed in terms of an appropriate equivalent circuit using the ZSimpWin program, and the values of the parameters were determined by the simulation. In the circuit, capacitance was mathematically modeled using a constant phase element Q in order to obtain a better simulation between the model and the experimental data. Then, the impedance was defined by [13,14]
 (1)
(1)
where Y0 is the Q-constant; j is the imaginary unit; ω is the angular frequency (ω=2πf, f is the frequency); n the Q-power ranging from -1 to 1.
2.4 EN measurements
Electrochemical noise data of 7A60 alloy in 3.5% NaCl solution was measured by using two nominally identical 7A60 alloys with an exposed area of 2 cm2 as working electrodes and a saturated calomel electrode as reference electrode. The EN system used was specifically described in Ref. [15]. The sampling frequency used in this study was 2 Hz and each time record consisted of 512 s. The standard deviations (STD) of the potential and current noise were given to evaluate the corrosion activity. Before STD analysis, the direct current (DC) component was removed from the original EN data by a 5-order polynomial fitting every 512 s interval.
Wavelet transformation was also used to analyze EN signals [16]. By using the wavelet transformation technique based on orthogonal db2 wavelet, the collected electrochemical potential noise data were decomposed to seven levels (d1-d7) [17]. Then, for orthonormal discrete wavelet decomposition, the following power law was used [18,19]:
 (j=1, 2, …, 7) (2)
(j=1, 2, …, 7) (2)
where σ2 is the variance of EN signal; 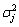 is the variance of detail crystal dj, which can be calculated by the following equation:
is the variance of detail crystal dj, which can be calculated by the following equation:
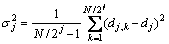 (j=1, 2, …, 7) (3)
(j=1, 2, …, 7) (3)
where N is the number of data record, k=1, 2, …, N/2j.
The slope β was obtained from the plot 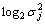 versus level j:
versus level j:
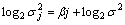 (4)
(4)
Finally, the fractal dimension D was obtained by
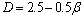 (5)
(5)
3 Results
3.1 Surface morphology
The microstructure and EDS spectra of 7A60 alloy before immersion are shown in Fig. 1. From Fig. 1 it can be observed that the round constituent particles were rich in Mg and Si elements (33.34% Mg and 15.7% Si in Fig. 1(b)), and could be related as β(Mg2Si) phase. The constituent particles with irregular shape were composed of 76.15% Al, 15.87% Cu and 7.98% Fe (Fig. 1(c)), and could be referred to as ternary phase Al7Cu2Fe. The oval particles should be S phase (Al2MgCu) with the composition of 50.17% Al, 24.59% Mg and 20.93% Cu (Fig. 1(d)). The particles with high contents of Mg and Zn in Fig. 1(e) could be related as η phase (MgZn2) [9,12,20].
The surface morphology of 7A60 alloy after immersion is shown in Fig. 2. During the initial 2 h of immersion, there were no visible pits. However, it was clearly seen that the oxide layer rupture (pits nucleation) was mainly localized at the constituent particles (Fig. 2(a)). As immersion time increased to 3 h, the corrosion pits with an average size of 20 μm were observed, and the particles in pits rich in Mg and Zn could be related as MgZn2 phase (Fig. 2(b)). After 10 h of immersion, some stable pits grew up and the alloy surface was covered with corrosion product of mud structure (Fig. 2(c)). For up to 48 h and 72 h of immersion, the size and depth of corrosion pits were further increased with the diameter of 100 μm for 48 h and 200 μm for 72 h (Figs. 2(d) and (e)).

Fig. 1 SEM image (a) and EDS spectra (b-e) of main existing constituent particles in 7A60 samples
The magnified morphologies of corrosion pits induced by constituent particles are shown in Fig. 3, and the EDS spectra of the related constituent particles are also shown in Fig. 3. Around Al7Cu2Fe particles, the size of corrosion pits was much larger than that of the particles. It indicated that during the corrosion process the surrounding Al matrix was dissolved as anode whereas Al7Cu2Fe particle was cathode (Fig. 3(a)). And EDS result revealed that the Al7Cu2Fe particles were stable (Fig. 3(b)).
For Al2MgCu particle, obvious corrosion trenches at the interface of Al2MgCu particles and Al matrix were observed (Fig. 3(c)). However, after corrosion the Al2MgCu particle was rich in Al and Cu, but Mg element was hardly detected (Fig. 3(d)). These results proved that preferential dissolution of Mg element occurred in the Al2MgCu particles during immersion test.
Apart from the above corrosion pits and constituents, the round constituent particle mainly composed of Si element was observed and anodic dissolution of Al matrix around the Si-rich particle was obvious (Figs. 3(e) and (f)). Based on a comparison with the Mg2Si particle shown in Figs. 1(a) and (b), it was indicated that very active Mg was selectively dissolved from Mg2Si, leaving Si-enriched remnants. It was similar to the dealloying of Al2MgCu particle [21,22].
As for MgZn2 particle, no corrosion pits containing MgZn2 particle were found. The possible reason was that MgZn2 particle was dissolved completely due to its anodic nature, and thus only deep corrosion pits left as shown in Figs. 2(d) and (e) [9].
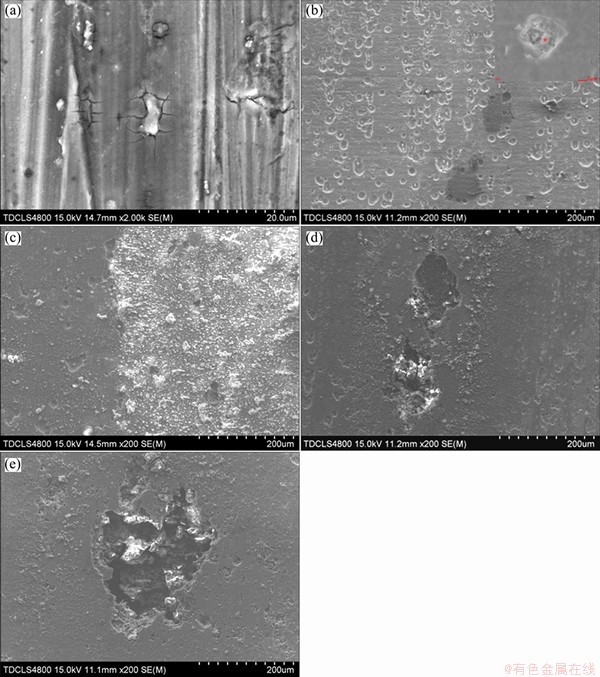
Fig. 2 SEM images of 7A60 alloy after immersion time of 2 h (a), 3 h (b), 10 h (c), 48 h (d) and 72 h (e) in 3.5% NaCl solution
3.2 EIS characterization
EIS plots of 7A60 alloy at different immersion time were determined and shown in Fig. 4. During the initial 2 h, EIS plots showed a capacitive impedance arc at high frequency and an inductive impedance arc at low frequency (Fig. 4(a)). The inductive arc could be related with pitting nucleation in the pitting model for Al alloys [13, 23]. With increasing immersion time to 3 h, 7A60 alloy had a capacitive impedance arc at high frequency and another capacitive impedance arc at low frequency, and the inductive impedance arc at low frequency disappeared (Fig. 4(b)). The capacitive arc at high frequency corresponded to the original surface while the one at low frequency was associated to the new generated surface in pits. Meanwhile, the radius of the capacitive arc at low frequency decreased with the increase of immersion time due to the anodic corrosion process in stable pits.
Figure 5 shows the phase angle plots of 7A60 alloy obtained from Fig. 4. During the initial 2 h, only one capacitive time constant was observed (Fig. 5(a)). After immersion for 2 h, two capacitive time constants could be found (Fig. 5(b)). Moreover, the phase angle peak at 0.01-0.1 Hz in Fig. 5(b) tended to depress with increasing time, which suggested that the pitting corrosion activity increased [14,24]. While the phase angle peak at 10-100 Hz showed no obvious tendency.
In order to analyze the EIS spectrum of 7A60 alloy, the corresponding equivalent circuits are shown in Fig. 6. In Fig. 6, Rsol represented solution resistance; Rt and Qp were defined as the charge transfer resistance and oxide-layer capacitance of the original flat surface (or the double-layer capacitance of the original corrosion product surface), respectively, while Rt(pit) and Qdl(pit) were the charge transfer resistance and double-layer capacitance corresponding to the new generated surface in pits, respectively; R0 and L were the inductive parameters.
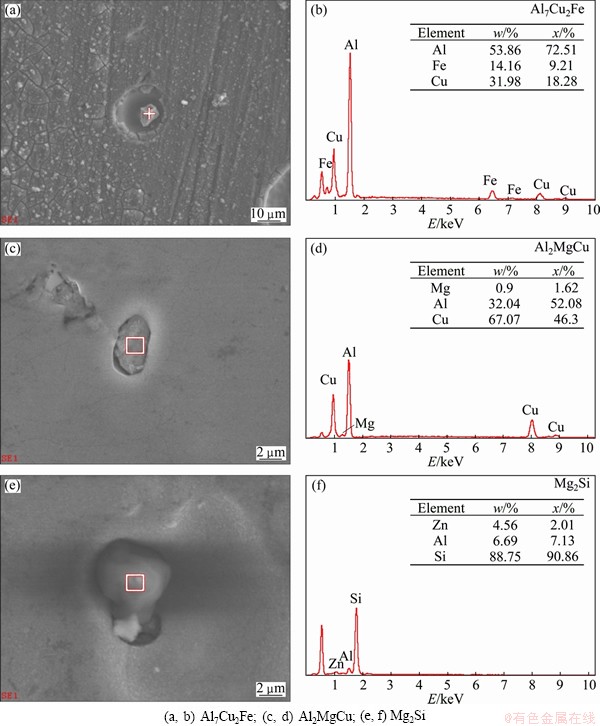
Fig. 3 Morphologies of corrosion pits and EDS spectra of constituent particles of 7A60 alloy
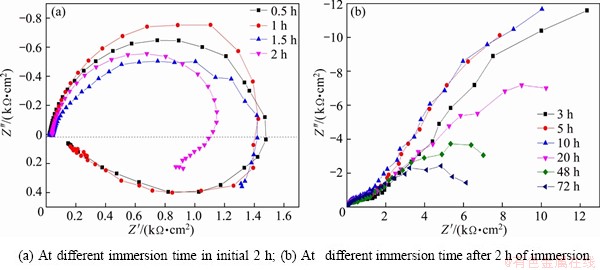
Fig. 4 Nyquist plots of 7A60 alloy during immersion in 3.5% NaCl solution
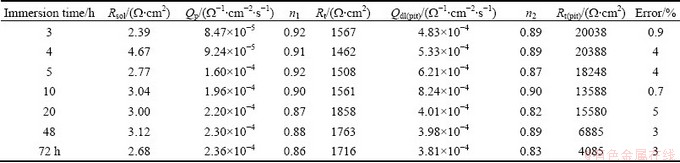
The fitting parameters of 7A60 alloy are listed in Tables 1 and 2. For a capacitance element, the derivation of n from the unit was due to the heterogeneous effect. n1 and n2 were around 0.9, showing a deviation from the ideal capacitive behavior. It could be attributed to the formation of an inhomogeneous corroded surface because of the dissolution of particles or Al matrix. And Rt increased slightly due to the formation of corrosion product on the metal surface [24,25]. After 2 h of immersion, Rt(pit) decreased markedly with increasing immersion time because of the propagation of stable pits in breadth and depth.
3.3 EN characterization
EN signals of 7A60 alloy in 3.5% NaCl solution at different immersion time are shown Fig. 7. During the initial 2 h of immersion, the potential and current noise signals showed high frequency fluctuations consisted of lots of overlapped transients with low amplitude (Fig. 7(a)). It illustrated that the oxide layer rupture of 7A60 alloy around the constituent particles occurred on the whole surface, and no visible pits could be observed (Fig. 2(a)). At the immersion time of 3 h, the potential and current noise signals showed low frequency fluctuations consisted of some transients with a long time width, and the potential amplitude was an order of magnitude higher than that of the initial 2 h (Fig. 7(b)). It indicated the onset of a pitting type of corrosion on the active sites (Fig. 2(b)). When immersion time was prolonged to 10 and 72 h, the EN signals all showed low frequency fluctuations with large transients, and the current amplitude also increased (Figs. 7(c) and (d)). It was corresponding to the development of corrosion pits on the alloy surface (Figs. 2(c)-(e)).
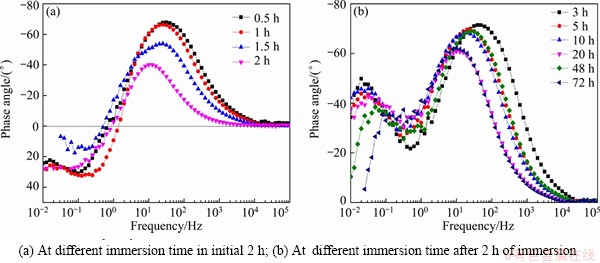
Fig. 5 Phase angle plots of 7A60 alloy during immersion in 3.5% NaCl solution
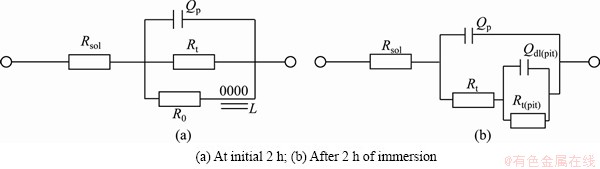
Fig. 6 Equivalent circuit of 7A60 alloy
Table 1 EIS fitting parameters of 7A60 alloy at different immersion time in initial 2 h
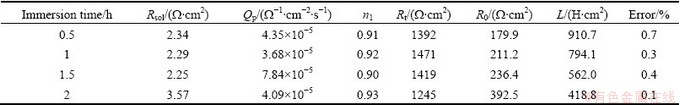
Table 2 EIS fitting parameters of 7A60 alloy at different immersion time after 2 h of immersion
The STD of potential (STDφ) and current noise (STDI), and the wavelet fractal dimension D were calculated and shown in Fig. 8. During the initial 2 h, The STDφ and STDI were relatively small, and the fractal dimension D was in the range of 1.7-1.8. With the increase of immersion time, the STDφ and STDI of 7A60 alloy increased significantly (Fig. 8(a)) [26,27], whereas the fractal dimension D decreased steeply and then reached a constant value around 1.4 (Fig. 8(b)).
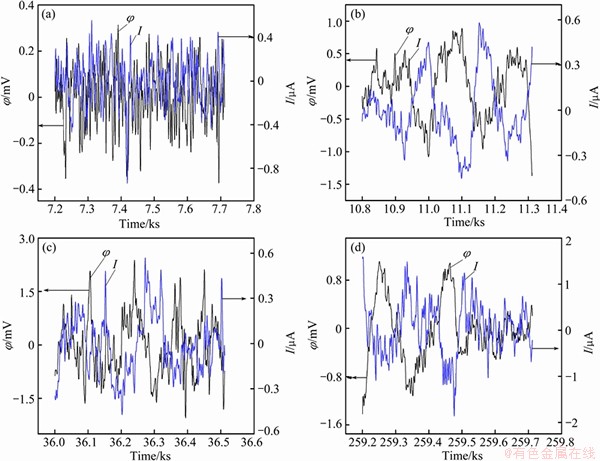
Fig. 7 EN signals of 7A60 alloy in 3.5% NaCl solution at immersion time of 2 h (a), 3 h (b), 10 h (c) and 72 h (d) after DC removal
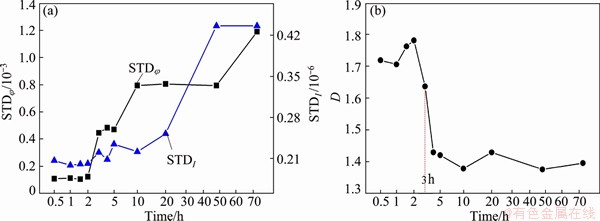
Fig. 8 Variation of STDφ and STDI (a), and fractal dimension D (b) of 7A60 alloy with immersion time
4 Discussion
4.1 Effect of constituent particles
The microstructures of 7xxx series aluminum alloys incorporate a combination of constituent particles: Mg2Si, MgZn2, Al7Cu2Fe, Al2Cu, Al2MgCu and Al3Fe [12]. The constituent particles did not precipitate for the purpose of strength development specifically. Generally, these particles lead to rather severe micro-galvanic corrosion in NaCl solution, as they are rich in alloying elements and their electrochemical behavior is significantly different from the surrounding Al matrix [28]. The main constituent particles in 7A60 alloy are η(MgZn2), S(Al2MgCu), β(Mg2Si) and Al7Cu2Fe (Fig. 1). They exhibit either anodic or cathodic characteristics relative to the matrix. Since these particles can be formed during alloy solidification at high temperature, they are unaffected by the subsequent aging treatment at low temperatures.
The corrosion potential of MgZn2 phase in 3.5% NaCl solution is -1095 mV (vs SCE) [12], while the corrosion potential of 7A60 alloy in this study is -767 mV (vs SCE). Therefore, η(MgZn2) phases are electrochemically active particles with high anodic dissolution ability. Before immersion, η(MgZn2) phases have high concentration of Mg and Zn elements (Figs. 1(a) and (e)). After immersion for 3 h, MgZn2 remnants in pits still can be seen (Fig. 2(b)). However, after 72 h of immersion, no MgZn2 particles can be observed because MgZn2 particles have been dissolved completely due to its anodic nature. It is reported that the dissolution of anodic particles can form pits which are very deep [29]. Pits of this type will propagate by self-catalyzed reaction [30]. As a result, the dissolution of anodic MgZn2 particles results in the occurrence of deep corrosion pits as shown in Figs. 2(d) and (e) [9].
The corrosion potentials of Al2MgCu and Mg2Si phases in 3.5% NaCl solution are -1061 and -1536 mV respectively [12]. As a result, S(Al2MgCu) and β(Mg2Si) particles are anodic relative to the Al matrix during the initial corrosion process, and they are rich in Mg (Figs. 1(b) and (d)). Nevertheless, the evidence of so-called peripheral matrix dissolution is observed around S(Al2MgCu) and β(Mg2Si) particles after 72 h of immersion (Figs. 3(c) and (e)). This is because dealloying and incongruent dissolution of Mg allow S(Al2MgCu) and β(Mg2Si) intermetallic particles to behave as a local cathode after some unknown time (Figs. 3(d) and (f)) [22,31].
Since Al7Cu2Fe particles are nobler than the aluminum matrix with corrosion potential of -654 mV in 3.5% NaCl solution [12], the aluminum matrix around Al7Cu2Fe particles is preferentially dissolved and the size of corrosion pits is larger than that of Al7Cu2Fe particles (Fig. 3(a)). Dissolution of the matrix around the cathodic particles continues as long as the particles retain electrical contact with the matrix [32]. Once the particles are detached from the pits, the corrosion process is stopped [33]. Therefore, the pits caused by Al7Cu2Fe can be re-passivated.
Consequently, the presence of the highly active η(MgZn2) is seen to make a very large contribution to the severe pitting corrosion damage, secondly S(Al2MgCu) and β(Mg2Si) [9].
4.2 Corrosion process
In general, there are two pitting corrosion stages for pitting corrosion of aluminum alloy: pitting initiation stage and pitting propagation stage. During the initial 2 h of immersion of 7A60 alloy in 3.5% NaCl solution, lots of oxide film rupture events are located around constituent particles (Fig. 2(a)). Consequently, EIS shows a capacitive arc at high frequency and an inductive arc at low frequency (Fig. 4(a)), the capacitive arc is an indication of the corrosion process for original surface under activation control, and the inductive behavior is attributed to the relaxation processes induced by Cl- ion adsorption [34].
From our previous study it can be deduced that the fractal dimension D can be used to distinguish the corrosion type and evaluate the localized degree of corrosion [35]. Since the oxide film rupture events are dispersed at lots of locations on the metal surface, and no pitting corrosion occurs, EN shows high frequency signal and high D value of about 1.7-1.8 (Figs. 7(a) and Fig. 8(b)).
As the immersion time increases, the inductive arc in EIS decreases and then disappears (Fig. 4). This indicates that the oxide layer is absent and the bare metal is attacked [36]. When the immersion time increases to 3 h, corrosion pits with a size of 20 μm can be observed (Fig. 2(b)). This process can be termed as pitting propagation stage. At the same time, EIS is characterized by double capacitive arcs (Fig. 4(b)). The capacitive arc at high frequency represents the oxide layer or corrosion products on metal surface, while the other at low frequency represents the new surface in pits [37]. Meanwhile, EN signal shows obvious large transients (Fig. 7(b)), the STDφ and STDI increase and D decreases due to the onset of local pitting corrosion (Fig. 8).
As the immersion time further increases to 10, 48 and 72 h, respectively, the stable pits are propagated in breadth and depth (Figs. 2(c)-(e)). Therefore, EN signal still shows obvious large transients, and the D value calculated from EN continues to decrease and reaches a steady state value of around 1.4 finally. This is in the range of D values for pitting corrosion (Fig. 8(b)) [35]. In addition, STDφ and STDI increase with immersion time for the enhanced anodic dissolution in deep pits (Fig. 8(a)). Meanwhile, for EIS, the increase of Rt (Tables 1 and 2) is ascribed to the corrosion products covered on the surface. And the decrease of Rt(pit) is caused by pitting propagation.
On the basis of the above discussion, the two pitting corrosion stages of 7A60 alloy in 3.5% NaCl solution can be distinguished by the appearance of two capacitive time constants in EIS and the wavelet fractal dimension D extracted from EN.
5 Conclusions
1) MgZn2 particles show high anodic dissolution ability. Preferential dissolution of Mg can occur in Al2MgCu and Mg2Si particles during immersion, showing evidence of dealloying. Al7Cu2Fe particles are noble and stable.
2) Severe pitting corrosion in 7A60 alloy is mainly induced by active MgZn2 particles, secondly Al2MgCu and Mg2Si particles. And Al7Cu2Fe particles make little contribution to pitting corrosion.
3) At the pitting initiation stage of 7A60 alloy in 3.5% NaCl solution, EIS shows a capacitive arc at high frequency and an inductive arc at low frequency, and the fractal dimension D value extracted from EN is about 1.7-1.8. At the pitting propagation stage, EIS shows double capacitive arcs with D value of around 1.4.
References
[1] SONG F X, ZHANG X M, LIU S D, TAN Q, LI D F. Exfoliation corrosion behavior of 7050-T6 aluminum alloy treated with various quench transfer time [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(7): 2258-2265.
[2] REDA Y, ABDEL-KARIM R, ELMAHALLAWI I. Improvements in mechanical and stress corrosion cracking properties in Al-alloy 7075 via retrogression and reaging [J]. Materials Science and Engineering A, 2008, 485(1-2): 468-475.
[3] TANAKA H, MINODA T. Mechanical properties of 7475 aluminum alloy sheets with fine subgrain structure by warm rolling [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(7): 2187-2195.
[4] BAYDOGAN M, CIMENOGLU H, SABRI KAYALI E, RASTY J. Improved resistance to stress-corrosion-cracking failures via optimized retrogression and reaging of 7075-T6 aluminum sheets [J]. Metallurgical and Materials Transactions A, 2008, 39(10): 2470-2476.
[5] ANGAPPAN M, SAMPATH V, ASHOK B, DEEPKUMAR V P. Retrogression and re-aging treatment on short transverse tensile properties of 7010 aluminium alloy extrusions [J]. Materials & Design, 2011, 32(7): 4050-4053.
[6] CHEN S Y, CHEN K H, DONG P X, YE S P, HUANG L P. Effect of heat treatment on stress corrosion cracking, fracture toughness and strength of 7085 aluminum alloy [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(7): 2320-2325.
[7] WANG X H, WANG J H, SONG J, FU C W. Effect of retrogression time on the mechanical properties and stress corrosion cracking behavior of 7A60 aluminum alloy [J]. Advanced Materials Research, 2012, 562-564: 227-233.
[8] LIU X F, ZHAN J, LIU Q J. The influence of tensile stress on electrochemical noise from aluminum alloy in chloride media [J]. Corrosion Science, 2009, 51(6): 1460-1466.
[9] XU D K, BIRBILIS N, LASHANSKY D, ROMETSCH P A, MUDDLE B C. Effect of solution treatment on the corrosion behaviour of aluminium alloy AA7150: Optimisation for corrosion resistance [J]. Corrosion Science, 2011, 53(1): 217-225.
[10] YUE T M, DONG C F, YAN L J, MAN H C. The effect of laser surface treatment on stress corrosion cracking behaviour of 7075 aluminium alloy [J]. Materials Letters, 2004, 58(5): 630-635.
[11] SRINIVASAN P B, DIETZEL W, ZETTLER R, DOS SANTOS J F, SIVAN V. Stress corrosion cracking susceptibility of friction stir welded AA7075-AA6056 dissimilar joint [J]. Materials Science and Engineering A, 2005, 392(1-2): 292-300.
[12] BIRBILIS N, BUCHHEIT R G. Electrochemical characteristics of intermetallic phases in aluminum alloys: An experimental survey and discussion [J]. Journal of the Electrochemical Society, 2005, 152(4): B140-B151.
[13] JINGLING M A, JIUBA W, GENGXIN L I, CHUNHUA X V. The corrosion behaviour of Al-Zn-In-Mg-Ti alloy in NaCl solution [J]. Corrosion Science, 2010, 52(2): 534-539.
[14] DENG Y, YIN Z M, ZHAO K, DUAN J Q, HU J, HE Z B. Effects of Sc and Zr microalloying additions and aging time at 120 °C on the corrosion behaviour of an Al-Zn-Mg alloy [J]. Corrosion Science, 2012, 65: 288-298.
[15] DU G, LI J, WANG W K, JIANG C, SONG S Z. Detection and characterization of stress-corrosion cracking on 304 stainless steel by electrochemical noise and acoustic emission techniques [J]. Corrosion Science, 2011, 53(9): 2918-2926.
[16] SAFIZADEH F, GHALI E. Electrochemical noise of copper anode behaviour in industrial electrolyte using wavelet analysis [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(6): 1854-1862.
[17] CAO F H, ZHANG Z, SU J X, SHI Y Y, ZHANG J Q. Electrochemical noise analysis of LY12-T3 in EXCO solution by discrete wavelet transform technique [J]. Electrochimica Acta, 2006, 51(7): 1359-1364.
[18] LIU X F, WANG H G, GU H C. Fractal characteristic analysis of electrochemical noise with wavelet transform [J]. Corrosion Science, 2006, 48(6): 1337-1367.
[19]  P, PETEK A. Characterization of corrosion processes by current noise wavelet-based fractal and correlation analysis [J]. Electrochimica Acta, 2008, 53(16): 5206-5214.
P, PETEK A. Characterization of corrosion processes by current noise wavelet-based fractal and correlation analysis [J]. Electrochimica Acta, 2008, 53(16): 5206-5214.
[20] MALJUSCH A,  C, ROHWERDER M, SCHUHMANN W. Combined high resolution scanning kelvin probe-scanning electrochemical microscopy investigations for the visualization of local corrosion processes [J]. Electrochimica Acta, 2012, 82: 339-348.
C, ROHWERDER M, SCHUHMANN W. Combined high resolution scanning kelvin probe-scanning electrochemical microscopy investigations for the visualization of local corrosion processes [J]. Electrochimica Acta, 2012, 82: 339-348.
[21] BUCHHEIT R G, MONTES L P, MARTINEZ M A, MICHAEL J, HLAVA P F. The electrochemical characteristics of bulk-synthesized Al2CuMg [J]. Journal of the Electrochemical Society, 1999, 146(12): 4424-4428.
[22] YASAKAU K A, ZHELUDKEVICH M L, LAMAKA S V, FERREIRA M G S. Role of intermetallic phases in localized corrosion of AA5083 [J]. Electrochimica Acta, 2007, 52(27): 7651-7659.
[23] CAO C N, WANG J, LIN H C. Effect of Cl- ion on the impedance of passive-film-covered electrodes [J]. Journal of Chinese Society of Corrosion and Protection, 1989, 9(4): 261-270. (in Chinese)
[24] YE C Q, HU R G, DONG S G, ZHANG X J, HOU R Q, DU R G, LIN C J, PAN J S. EIS analysis on chloride-induced corrosion behavior of reinforcement steel in simulated carbonated concrete pore solutions [J]. Journal of Electroanalytical Chemistry, 2013, 688: 275-281.
[25] LOU X Y, SINGH P M. Phase angle analysis for stress corrosion cracking of carbon steel in fuel-grade ethanol: Experiments and simulation [J]. Electrochimica Acta, 2011, 56(4): 1835-1847.
[26] KLAPPER H S, GOELLNER J, HEYN A. The influence of the cathodic process on the interpretation of electrochemical noise signals arising from pitting corrosion of stainless steels [J]. Corrosion Science, 2010, 52(4): 1362-1372.
[27] KLAPPER H S, GOELLNER J. Electrochemical noise from oxygen reduction on stainless steel surfaces [J]. Corrosion Science, 2009, 51(1): 144-150.
[28] BIRBILIS N, CAVANAUGH M K, BUCHHEIT R G. Electrochemical behavior and localized corrosion associated with Al7Cu2Fe particles in aluminum alloy 7075-T651 [J]. Corrosion Science, 2006, 48(12): 4202-4215.
[29]  M, WATARI T, SMYRL W H. Investigation of the initiation of localized corrosion on aluminum alloys by using fluorescence microscopy [J]. Corrosion Science, 2000, 42(9): 1661-1668.
M, WATARI T, SMYRL W H. Investigation of the initiation of localized corrosion on aluminum alloys by using fluorescence microscopy [J]. Corrosion Science, 2000, 42(9): 1661-1668.
[30] XU W F, LIU J H. Microstructure and pitting corrosion of friction stir welded joints in 2219-O aluminum alloy thick plate [J]. Corrosion Science, 2009, 51(11): 2743-2751.
[31] LEBLANC P, FRANKEL G S. A study of corrosion and pitting initiation of AA2024-T3 using atomic force microscopy [J]. Journal of the Electrochemical Society, 2002, 149(6): B239-B247.
[32] QAFSAOUI W, HUET F, TAKENOUTI H. Analysis of the inhibitive effect of BTAH on localized corrosion of Al 2024 from electrochemical noise measurements [J]. Journal of the Electrochemical Society, 2009, 156(2): C67-C74.
[33] WANG X H, WANG J H, SONG J, FU C W. Effect of annealing treatment on the mechanical properties and corrosion behaviors of 01570 aluminum alloy [J]. Materials and Corrosion, 2014, 65(8): 809-814.
[34] JIANG Q, MIAO Q, TONG F, XU Y, REN B L, LIU Z M, YAO Z J. Electrochemical corrosion behavior of arc sprayed Al-Zn-Si-RE coatings on mild steel in 3.5% NaCl solution [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(8): 2713-2722.
[35] WANG X H, WANG J H, FU C W, GAO Y K. Determination of corrosion type by wavelet-based fractal dimension from electrochemical noise [J]. International Journal of Electrochemical Science, 2013, 8: 7211-7222.
[36] CONDE A, de DAMBORENEA J. Electrochemical modelling of exfoliation corrosion behaviour of 8090 alloy [J]. Electrochimica Acta, 1997, 43(8): 849-860.
[37] WANG J, CAO C N, LIN H C. Features of AC impedance of pitting corroded electrodes during pits propagation [J]. Journal of Chinese Society for Corrosion and Protection, 1989, 9(4): 271-279. (in Chinese).
王学慧1, 2,王吉会1, 2,付丛伟2
1. 天津大学 水利工程仿真与安全国家重点实验室,天津 300072;
2. 天津大学 材料科学与工程学院,天津复合与功能材料重点实验室,天津 300072
摘 要:采用电化学阻抗谱(EIS)和电化学噪声(EN)方法研究回归再时效(RRA)热处理状态下7A60铝合金的点蚀行为,通过扫描电子显微镜(SEM)和能谱仪(EDS)观察和分析合金的组织和第二相颗粒成分。结果表明,7A60铝合金在3.5% NaCl溶液中存在两个腐蚀阶段,并且可以用EIS出现两个电容时间常数的时间和由EN计算出的小波分形维数D的变化来表征。SEM和EDS分析结果表明,在7A60铝合金中,严重的点蚀主要是由阳极相MgZn2引起的,其次是Al2MgCu和 Mg2Si相,Al7Cu2Fe 相对7A60铝合金点蚀行为的影响不大。
关键词:7A60铝合金;金属间化合物;点蚀;电化学阻抗谱;电化学噪声;小波分形维数
(Edited by Wei-ping CHEN)
Foundation item: Project (13JCZDJC29500) supported by the Key Project of Tianjin Natural Science Foundation, China; Projects (2011CB610505, 2014CB046801) supported by the National Basic Research Program of China; Project (20120032110029) supported by the Specialized Research Fund for the Doctoral Program of Higher Education, China
Corresponding author: Ji-hui WANG; Tel/Fax: +86-22-27890010; E-mail: jhwang@tju.edu.cn
DOI: 10.1016/S1003-6326(14)63550-0

