低品位软锰矿碱浸预脱硅-流化焙烧制备锰酸钠
来源期刊:中国有色金属学报(英文版)2018年第5期
论文作者:邓祥意 冯雅丽 李浩然 杜竹玮 康金星 郭成林
文章页码:1045 - 1052
关键词:低品位软锰矿;脱硅;流化焙烧;锰酸钠
Key words:low-grade pyrolusite; desilication; fluidized roasting; sodium manganate
摘 要:采用低浓度碱浸对低品位软锰矿进行预脱硅处理,考察NaOH浓度、液固比、浸出温度、浸出时间及搅拌速率对硅浸出率的影响,研究碱浸过程动力学。结果表明:在NaOH起始浓度为20%、液固比为4:1、浸出温度为180 °C、浸出时间为4 h、搅拌速率为300 r/min的条件下,硅浸出率达到91.2%。缩核模型表明,碱浸过程受化学表面反应控制,其表观反应活化能为53.31 kJ/mol。通过正交试验对脱硅渣流化焙烧制备锰酸钠的条件进行优化,在硅浸出率为91.2%、NaOH/MnO2质量比为3:1、焙烧温度为500 °C、焙烧时间为4 h的条件下,锰酸钠的转化率为89.7%,且锰酸钠转化率随硅浸出率的升高而增加。
Abstract: Low concentration alkaline leaching was used for predesilication treatment of low-grade pyrolusite. The effects of initial NaOH concentration, liquid-to-solid ratio, leaching temperature, leaching time and stirring speed on silica leaching rate were investigated and the kinetics of alkaline leaching process was studied. The results show that silica leaching rate reached 91.2% under the conditions of initial NaOH concentration of 20%, liquid-to-solid ratio of 4:1, leaching temperature of 180 °C, leaching time of 4 h and stirring speed of 300 r/min. Shrinking-core model showed that the leaching process was controlled by the chemical surface reaction with activation energy Ea of 53.31 kJ/mol. The fluidized roasting conditions for preparation of sodium manganate were optimized by the orthogonal experiments using the desiliconized residue. The conversion rate of sodium manganate was obtained to be 89.7% under the conditions of silica leaching rate of 91.2%, NaOH/MnO2 mass ratio of 3:1, roasting temperature of 500 °C and roasting time of 4 h, and it increased with the increase of silicon leaching rate.
Trans. Nonferrous Met. Soc. China 28(2018) 1045-1052
Xiang-yi DENG1, Ya-li FENG1, Hao-ran LI2, Zhu-wei DU2, Jin-xing KANG1, Cheng-lin GUO1
1. School of Civil and Resource Engineering, University of Science and Technology Beijing, Beijing 100083, China;
2. State Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Science, Beijing 100090, China
Received 6 January 2017; accepted 21 July 2017
Abstract: Low concentration alkaline leaching was used for predesilication treatment of low-grade pyrolusite. The effects of initial NaOH concentration, liquid-to-solid ratio, leaching temperature, leaching time and stirring speed on silica leaching rate were investigated and the kinetics of alkaline leaching process was studied. The results show that silica leaching rate reached 91.2% under the conditions of initial NaOH concentration of 20%, liquid-to-solid ratio of 4:1, leaching temperature of 180 °C, leaching time of 4 h and stirring speed of 300 r/min. Shrinking-core model showed that the leaching process was controlled by the chemical surface reaction with activation energy Ea of 53.31 kJ/mol. The fluidized roasting conditions for preparation of sodium manganate were optimized by the orthogonal experiments using the desiliconized residue. The conversion rate of sodium manganate was obtained to be 89.7% under the conditions of silica leaching rate of 91.2%, NaOH/MnO2 mass ratio of 3:1, roasting temperature of 500 °C and roasting time of 4 h, and it increased with the increase of silicon leaching rate.
Key words: low-grade pyrolusite; desilication; fluidized roasting; sodium manganate
1 Introduction
Manganese is one of the significant strategic resources, which plays an important role in many fields, such as ferrous metallurgy, nonferrous metal production [1], battery production [2] and fine chemicals [3]. Sodium manganate (Na2MnO4) is an analogue of K2MnO4 and can be obtained by chemical reaction of MnO2 and sodium hydroxide (NaOH) [4]. Sodium manganate is similar to other manganates [5,6] in their chemical properties while NaOH used for Na2MnO4 preparation is much cheaper and more available than raw materials for other manganates production [7,8]. Sodium manganate is promising for practical applications as electrode material because of its low price and excellent cycling behavior in the aqueous electrolyte without removing the dissolved oxygen [7,9].
The high-grade pyrolusite with considerable concentration of MnO2 is widely used for the preparation of potassium manganate and sodium manganate [10]. With the increasing demand for manganese resources and the shortage of high-grade pyrolusite, the exploitation of low-grade pyrolusite is becoming more and more urgent now [11,12]. However, the low-grade pyrolusite cannot be used for sodium manganate production directly because of the low concentration of MnO2 and the high ratio of SiO2.
In the preparation process of sodium manganate, SiO2 will react with NaOH to generate Na2O·nSiO2 [13-15] which affects the production purity of sodium manganate and predesilication is thus necessary. Desilication is a suitable method for improving the grade of the required elements. CHEN et al [16] reported the alkaline leaching behavior of desilication from titanium-vanadium slag. HUANG et al [17] studied pressure alkaline treatment for recovery of precious metals from spent auto catalysts. Up to now, the traditional physical mineral processing method cannot meet the requirements of the high grade and recovery of the product at the same time, while the chemical mineral processing mostly performs alkaline leaching in NaOH solution with mass fraction higher than 40% [14]. Moreover, high concentration solution of NaOH will corrode filter medium badly and could hardly be recovered and a large amount of acid is needed for the treatment of unreacted NaOH to prepare silicate products.
To improve the resource utilization efficiency and eliminate environmental pollution at the source, a high- efficiency method to prepare sodium manganate using low-grade pyrolusite is introduced in this research. This paper aims to study the leaching behavior of silica extracted from the low-grade pyrolusite and the effects of various leaching factors. Another object is to find the relationship between silica leaching rate and conversion rate of sodium manganate, and obtain the material with high manganese and low silica contents for preparation of sodium manganate by removing silica as much as possible. The shrinking-core model was introduced to indicate the silica leaching kinetics of the low-grade pyrolusite and provide theoretical guidance for exploring the leaching process.
2 Experimental
2.1 Materials
The low-grade pyrolusite sample was provided from Yunnan Province, China. The ore sample was crushed and ground into powder with the particle size smaller than 147 μm. The chemical multi-elemental analysis (as listed in Table 1) shows that the low-grade pyrolusite ore is mainly composed of 60.35% SiO2 and 28.89% MnO2. The analysis also reveals that there are other impurities such as 3.89% Al2O3, 5.68% Fe2O3, 0.27% MgO, 0.71% CaO, 0.19% V2O5 in pyrolusite. The mineralogical composition of the ore sample was analyzed by powder X-ray diffraction (XRD). The XRD pattern (Fig. 1) shows that the main metallic minerals include manganese dioxide, hematite, and the main gangue mineral is quartz. The sample of the low-grade pyrolusite was analyzed by scanning electron microscopy (SEM) with energy dispersive spectroscopy (EDS) to support the mineral characterization, which is presented in Fig. 2. It reveals that MnO2 is finely disseminated and intimately associated with SiO2, and MnO2 cannot be “liberated” with traditional methods efficiently.
Table 1 Chemical composition of low-grade pyrolusite (mass fraction, %)

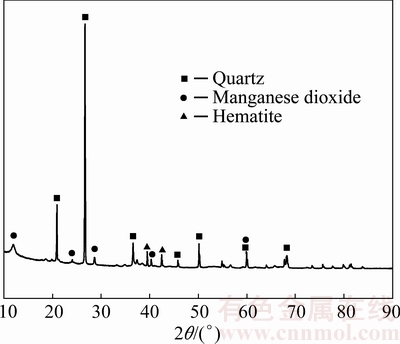
Fig. 1 XRD pattern of low-grade pyrolusite
2.2 Experimental procedure
Leaching experiments were carried out in titanium alloy autoclaves with a capacity of 500 mL. The flow chart of the process is shown in Fig. 3. The procedure is as follows: after crushing, rod milling, screening, and drying, 40 g of pyrolusite and certain amount of NaOH solution were added into the reaction reactor while the stove was heated to the setting temperature rapidly. The autoclaves were tightly sealed, stirred and maintained at 150-190 °C. Then, the pulp was rapidly cooled down to 40 °C, and the silica content in leaching solution was analyzed with molybdosilicate yellow spectrophoto- metry [18]. Afterwards, the desiliconized pyrolusite and NaOH with a certain mass ratio were separately added into quartz fluidized bed reactor. Then, a certain amount of air flow was blown into the reactor to make sure that the feed layer can be fluidized well. After roasting, the products were put into 10% NaOH (mass fraction) solution, then the solution containing sodium manganate could be obtained and the undissolved residue was filtrated. All the experiments were carried out independently in triplicate and repeated at least twice.
3 Results and discussion
3.1 Desilication leaching under high pressure
The desilication process was carried out using various concentrations of NaOH and the overall chemical reaction was assumed as follows:
nSiO2(s)+2NaOH(aq)→Na2O·nSiO2(aq)+H2O(l) (1)
3.1.1 Effect of initial NaOH concentration
The effect of initial NaOH concentration on the silica leaching rate was examined under the conditions of the liquid-to-solid ratio of 4:1, the stirring speed of 300 r/min, and leaching temperature of 180 °C. As shown in Fig. 4, the silica leaching rate increases with increasing the initial NaOH concentration from 10% to 30%. However, the enhancement of desilication is indistinctive when the initial NaOH concentration is increased to over 20%, and the residual NaOH without reaction could affect the follow-up process, so the suitable initial NaOH concentration was chosen as 20%.
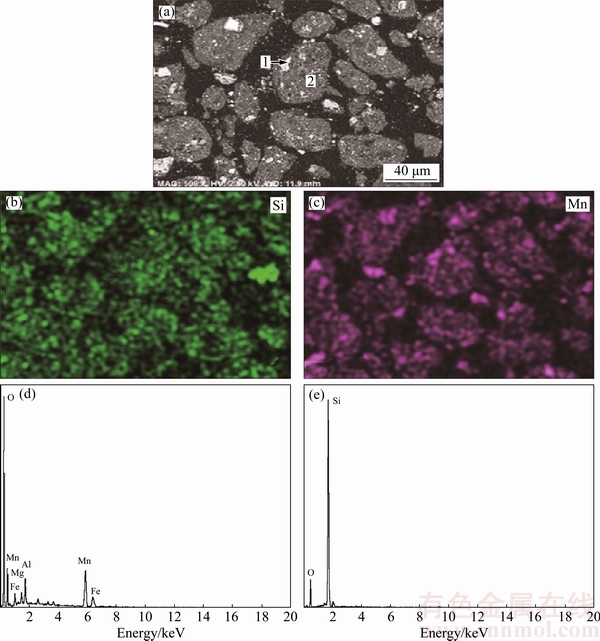
Fig. 2 SEM image of low-grade pyrolusite (a), EDS element mappings of Si (b), Mn (c) and EDS spectra of particles 1 (d) and 2 (e) in (a)

Fig. 3 Principle flow chart of low-grade pyrolusite for preparation of sodium manganate
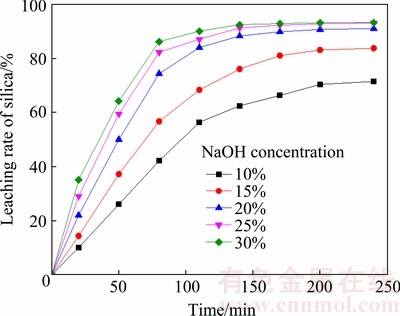
Fig. 4 Effect of initial NaOH concentration on leaching rate of silica
3.1.2 Effect of liquid-to-solid mass ratio (L/S ratio)
As shown in Fig. 5, the effect of liquid-to-solid ratio on the leaching rate of silica was studied at the ratios of 2:1, 3:1, 4:1 and 5:1. All the experiments were performed under the conditions of the initial NaOH content of 20%, the stirring speed of 300 r/min and the leaching temperature of 180 °C. The results showed that the leaching rate of silica increased from 74.2% to 92.5% with the liquid-to-solid ratio rising from 2:1 to 5:1. The increasing liquid-to-solid ratio favored the silica leaching rate. The higher the liquid-to-solid ratio is, the higher the viscosity of the solution is and the more the remaining NaOH in the solution is, thus silica will be released more easily.
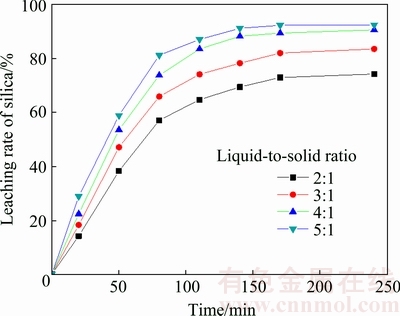
Fig. 5 Effect of liquid-to-solid ratio on leaching rate of silica
3.1.3 Effect of stirring speed
Experiments with various stirring speeds were carried out under typical test conditions of the initial NaOH concentration of 20%, the liquid-to-solid ratio of 4:1 and the leaching temperature of 180 °C. According to the results shown in Fig. 6, the influence of the stirring speed on the leaching process is not as significant as that of other parameters. Because the bubbles produced in leaching solution system were enhanced under high pressure [19] and thus reduced the efficiency of stirring, the silica leaching rate does not have a significant change with increasing the stirring speed from 300 to 500 r/min.
3.1.4 Effect of leaching temperature
The effect of leaching temperature on the leaching rate of silica was investigated at 150, 160, 170, 180 and 190 °C with the initial NaOH concentration of 20%, the liquid-to-solid ratio of 4:1 and the stirring speed of 300 r/min. According to the results shown in Fig. 7, the leaching temperature has a remarkable influence on the silica leaching rate. As the temperature increases, the silica leaching rate increases from 68.5% (at 150 °C) to 93.7% (at 190 °C), and the time of reaction equilibrium becomes shorter with a higher leaching temperature. Because of the exponential dependence of the rate constant in the Arrhenius equation, the chemical reaction is accelerated and the leaching rate is also improved with the increase of temperature.
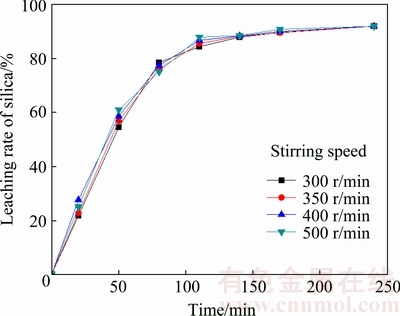
Fig. 6 Effect of stirring speed on leaching rate of silica
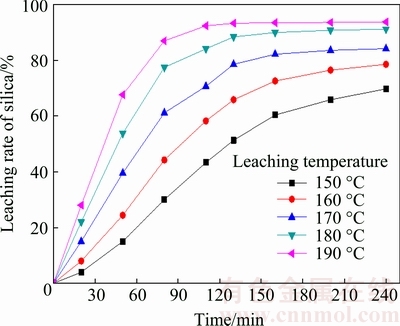
Fig. 7 Effect of leaching temperature on leaching rate of silica
3.1.5 Kinetic analysis
As shown in Eq. (1), during the leaching silica reaction there is no solid generated, so the mass of solid and the particle size are reduced during the leaching reaction. The shrinking-core model can be chosen to describe the leaching kinetics [20,21]. The heterogeneous reaction at the fluid/solid boundary can be generally controlled by one of the following steps: diffusion through the ash, diffusion through the fluid film or chemical reaction on the surface of the core [22]. If no ash layer covers the un-reacted core in the reaction process, there are only two controlling steps: chemical surface reaction or fluid film diffusion.
If the process is controlled by the resistance of chemical surface reaction, Eq. (2) can be used to represent the process [23]:
1-(1-x)1/3=k0t (2)
where x is the leaching rate of silica, k0 is the reaction rate constant, and t is the reaction time.
If the process is controlled by fluid film diffusion of no product layer, Eq. (3) can be used to represent the process [24]:
1-(1-x)2/3=k0t (3)
where k0=BmBKcA/ρBRB, B is a constant, mB is the molar mass of pyrolusite, K is the diffusion constant, cA is the concentration of NaOH, ρB is the density of pyrolusite, RB is the radius of pyrolusite particles.
To confirm the controlling step of the leaching process, all the experimental data were analyzed and multiple regression coefficients obtained for the integral rate expression were calculated with Eq. (2) and Eq. (3). Only Eq. (2) fits well with the experimental data shown in Fig. 8. The results are listed in Table 2. It indicates that the leaching silica reaction is controlled by the resistance of chemical surface reaction preliminary.
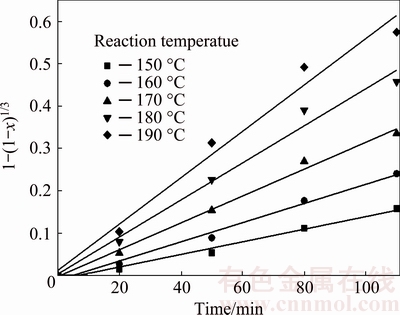
Fig. 8 Plots of leaching kinetics under various reaction temperatures
Table 2 Data of leaching kinetics at different temperatures
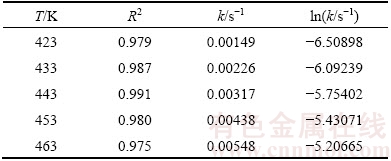
The effect of temperature on the leaching kinetics can be characterized by the value of the activation energy. The process is controlled by chemical surface reaction when the activation energy values are higher than 40 kJ/mol, whereas it is controlled by fluid diffusion when the activation energy values are smaller than 20 kJ/mol.
The Arrhenius equation can be written as
ln k=-Ea/(RT)+ln A (4)
where Ea denotes the experimental activation energy (kJ/mol), R is the molar gas constant (8.314 J/(mol·K)), T is the temperature (K), and A is a pre-exponential term. As shown in Fig. 9, a plot of ln k versus 1/T, in which k is determined by Eq. (2), is a straight line where the slope is -Ea/R. The value of -Ea/R is -6412.15, hence, the experimental activation energy value is 53.31 kJ/mol, which is consistent with the target of activation energy controlled by chemical surface reaction.
Consequently, Eq. (2) can be rewritten as
1-(1-x)1/3=t·exp(-6412.15/T+8.691) (5)
In conclusions, the kinetics of leaching silica reaction can be described by the shrinking-core model with the chemical surface reaction controlling.
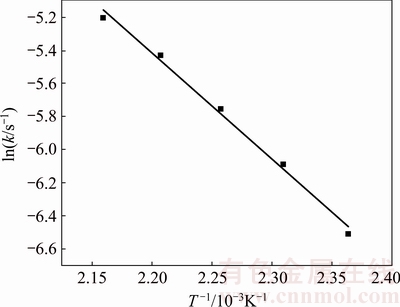
Fig. 9 Relationship between ln k and 1/T
3.1.6 Leaching behaviors of silica and manganese
The leaching behaviors of silica and manganese were investigated under the conditions of leaching temperature of 180 °C, liquid-to-solid ratio of 4:1, stirring speed of 300 r/min and initial NaOH concentration of 20%. Figure 10 shows the leaching rates of Si and Mn during the leaching process. It can be seen that the leaching rate of silica is 91.2% while the leaching rate of manganese is only 2.1% after 4 h leaching. The SEM-EDS analysis results of the desiliconized residue are presented in Fig. 11. Compared with the EDS mapping of Si and Mn in Fig. 2, it is obvious that the majority of silica was dissolved by the alkaline leaching process, most of MnO2 was “liberated” from the surrounding SiO2 and the undissolved residue with low silica content could be obtained for the preparation of sodium manganate.

Fig. 10 Leaching behaviors of Mn and Si during leaching process
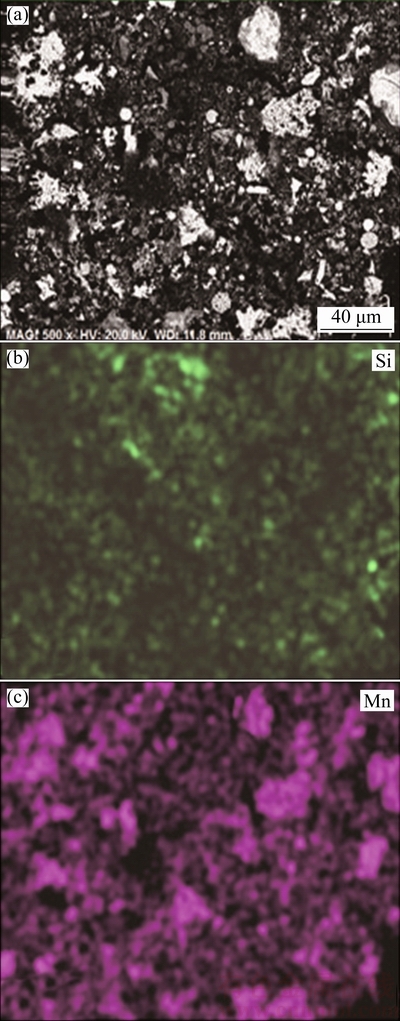
Fig. 11 SEM image of low-grade pyrolusite (a) and EDS mappings of Si (b) and Mn (c)
3.2 Preparation of sodium manganate by fluidized roasting
The main reaction of the process for preparing sodium manganate by fluidized roasting can be assumed as follows:
2MnO2(s)+4NaOH(s)+O2(g)=2Na2MnO4(s)+2H2O(g) (6)
In order to obtain the optimum process conditions of fluidized roasting for the preparation of sodium manganate with the desiliconized residue, the influences of silica leaching rate, the mass ratio of alkali to manganese dioxide, the roasting temperature and the roasting time were investigated respectively through orthogonal experimental design. An L9(34) matrix, which is an orthogonal array of four factors and three levels [25], was applied to assigning the considered factors and levels, and the results are shown in Table 3. The data analysis was carried out through range analysis and the optimal reaction conditions could be found by analysis of variance, as listed in Tables 4 and 5, respectively.
Table 3 Conversion rate of sodium manganate in L9(34) matrix
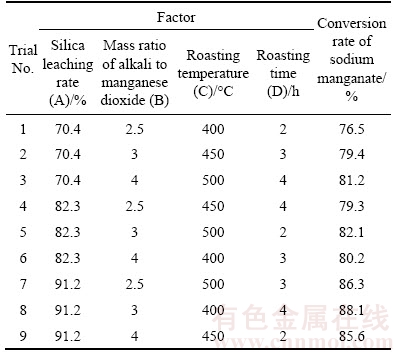
Table 4 Range analysis results of conversion rate of sodium manganate
Table 5 Variance analysis of conversion rate of sodium manganate

It was found that the effect of silica leaching rate on the conversion rate of sodium manganate is significant. By analyzing the data listed in Tables 4 and 5, the order of significant factors for the preparation of sodium manganate is: silica leaching rate > mass ratio of alkali to manganese dioxide > roasting temperature > roasting time. After the orthogonal experiments and the range analysis, the optimal level for each factor was determined as follows: silica leaching rate, 91.2%; the mass ratio of alkali to manganese dioxide, 3:1; the roasting temperature, 500 °C and the roasting time, 4 h.
In order to verify the effect of silica leaching rate on the conversion rate of sodium manganate, experiments were carried out under conditions of mass ratio of alkali to manganese dioxide of 3:1, roasting temperature of 500 °C, and roasting time of 4 h. According to the results shown in Fig. 12, the conversion rate of sodium manganate increases steadily with increasing the silica leaching rate. As the silica leaching rate rises from 0 to 91.2%, the conversion rate of sodium manganate increases from 20.3% to 89.7%.
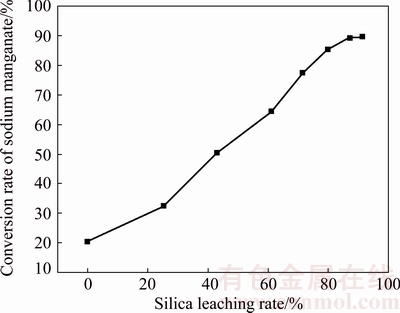
Fig. 12 Effect of silica leaching rate on conversion rate of sodium manganate
4 Conclusions
1) Silica leaching rate was obtained to be 91.2% under the conditions of the initial NaOH concentration of 20%, the liquid-to-solid ratio of 4:1, and the stirring speed of 300 r/min, the leaching temperature of 180 °C and the leaching time of 4 h.
2) The kinetics of alkaline predesilication process was investigated at various parameter levels. The leaching process obeys a shrinking-core model controlled by the chemical surface reaction with the activation energy of 53.31 kJ/mol, and the kinetics equation was established.
3) The majority of MnO2 surrounded by SiO2 was “liberated” by alkaline predisilication treatment. In the fluidized roasting process, the conversion rate of sodium manganate increased with the increase of silicon leaching rate and the conversion rate of sodium manganate was obtained to be 89.7% under the conditions of silica leaching rate of 91.2%, NaOH/MnO2 mass ratio of 3:1, roasting temperature of 500 °C and roasting time of 4 h.
References
[1] TANG Qing, ZHONG Hong, WANG Shuai, LI Jin-zhong, LIU Guang-yi. Reductive leaching of manganese oxide ores using waste tea as reductant in sulfuric acid solution [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(3): 861-867.
[2] ZHAO Yu-qian, JIANG Qing-lai, WANG Wei-gang, DU Ke, HU Guo-rong. Effect of electrolytic MnO2 pretreatment on performance of as-prepared LiMn2O4 [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(5): 1146-1150.
[3] SU Hai-feng, WEN Yan-xuan, WANG Fan, SUN Ying-yun, TONG Zhang-fa. Reductive leaching of manganese from low-grade manganese ore in H2SO4 using cane molasses as reductant [J]. Hydrometallurgy, 2008, 93(3-4): 136-139.
[4] REIDIES A H. Manganese compounds [M]. Porsgrunn: Ullmann’s Encyclopedia of Industrial Chemistry, 2000.
[5] CHEN R J, CHIRAYIL T, ZAVALIJ P, WHITTINGHAM M. The hydrothermal synthesis of sodium manganese oxide and a lithium vanadium oxide [J]. Solid State Ionics, 1996, 86-88: 1-7.
[6] KAPPENSTEIN C, PIRAULT-ROY L,  M, WAHDAN T, ALI A A. Monopropellant decomposition catalysts: V. Thermal decomposition and reduction of permanganates as models for the preparation of supported MnOx catalysts [J]. Applied Catalysis A: General, 2002, 234(1-2): 145-153.
M, WAHDAN T, ALI A A. Monopropellant decomposition catalysts: V. Thermal decomposition and reduction of permanganates as models for the preparation of supported MnOx catalysts [J]. Applied Catalysis A: General, 2002, 234(1-2): 145-153.
[7] WANG Y, LI Z, LI H. A new process for leaching metal values from ocean polymetallic nodules [J]. Minerals Engineering, 2005, 18(11): 1093-1098.
[8] ZHANG B H, LIU Y, CHANG Z, YANG Y Q, WEN Z B, WU Y P, HOLZE R. Nanowire Na0.35MnO2 from a hydrothermal method as a cathode material for aqueous asymmetric supercapacitors [J]. Journal of Power Sources, 2014, 253(5): 98-103.
[9] WHITACRE J F, TEVAR A, SHARMA S. Na4Mn9O18 as a positive electrode material for an aqueous electrolyte sodium-ion energy storage device [J]. Electrochemistry Communications, 2010, 12(3): 463-466.
[10] PENG Dong, WANG Ji-kun. Reaction kinetics of potassium manganate prepared by three-phase compression oxidation process [J]. Journal of the Chinese Ceramic Society, 2012, 40(12): 1767-1772. (in Chinese)
[11] CAI Zhen-lei, FENG Ya-li, LI Hao-ran, DU Zhu-wei, LIU Xin-wei. Co-recovery of manganese from low-grade pyrolusite and vanadium from stone coal using fluidized roasting coupling technology [J]. Hydrometallurgy, 2013, 131-132(S1): s40-s45.
[12] SANTOS O D S H, CARVALHO C D F, SILVA G A, SANTOS C G D. Manganese ore tailing: Optimization of acid leaching conditions and recovery of soluble manganese [J]. Journal of Environmental Management, 2015, 147: 314-320.
[13] TOGNONVI M T, SORO J, GELET J L, ROSSIGNOL S. Physico-chemistry of silica/Na silicate interactions during consolidation. Part 2: Effect of pH [J]. Journal of Non-crystalline Solids, 2012, 358(3): 492-501.
[14] XIAO Qing-gui, CHEN Yin, GAO Yi-ying, XU Hong-bin, ZHANG Yi. Leaching of silica from vanadium-bearing steel slag in sodium hydroxide solution [J]. Hydrometallurgy, 2010, 104(2): 216-221.
[15] MA Jia-yu, ZHAI Kun-ming, LI Zhi-bao. Desilication of synthetic Bayer liquor with calcium sulfate dihydrate: Kinetics and modelling [J]. Hydrometallurgy, 2011, 107(1-2): 48-55.
[16] CHEN De-sheng, ZHAO Long-sheng, QI Tao, HU Guo-ping, ZHAO Hong-xin, LI Jie, WANG Li-na. Desilication from titanium– vanadium slag by alkaline leaching [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(10): 3076-3082.
[17] HUANG Kun, CHEN Jing, CHEN Yi-ran, ZHAO Jia-chun, LI Qi-wei, YANG Qiu-xue. Recovery of precious metals from spent auto-catalysts by method of pressure alkaline treatment-cyanide leaching [J]. Chinese Journal of Nonferrous Metals, 2006, 16(2): 363-369. (in Chinese)
[18] HE Huan, ZHANG Xu, SHEN Qing-feng, LI Xiao-ming, MENG Hong-li, LIU Hong-fei, WU Hai-yan. Determination of silicon dioxide in copper slag acid leaching solution with silicon molybdenum yellow spectrophotometry [J]. Chinese Journal of Analysis Laboratory, 2016, 35(2): 176-179. (in Chinese)
[19] HAN L, AL-DAHHAN M H. Gas–liquid mass transfer in a high pressure bubble column reactor with different sparger designs [J]. Chemical Engineering Science, 2007, 62(1-2): 131-139.
[20] NAYL A A, ISMAIL I M, ALY H F. Recovery of pure MnSO4·H2O by reductive leaching of manganese from pyrolusite ore by sulfuric acid and hydrogen peroxide [J]. International Journal of Mineral Processing, 2011, 100(3-4): 116-123.
[21] SANTOS F M F, PINA P S, PORCARO R, OLIVEIRA V A, SILVA C A, LEAO V A. The kinetics of zinc silicate leaching in sodium hydroxide [J]. Hydrometallurgy, 2010, 102(1-4): 43-49.
[22] LEVIEN K L, LEVENSPIEL O. Optimal product distribution from laminar flow reactors: Newtonian and other power-law fluids [J]. Chemical Engineering Science, 1999, 54(13-14): 2453-2458.
[23] WANG Wei-da, FENG Ya-li, LI Hao-ran, YANG Zhi-chao, ZHANG Xu. Recovering gold from cyanide residue by alkaline predesilication-cyanide leaching technique [J]. Chinese Journal of Nonferrous Metals, 2015, 25(1): 233-240. (in Chinese)
[24] WANG Ruo-chao, ZHAI Yu-chun, NING Zhi-qiang, MA Pei-hua. Kinetics of SiO2 leaching from Al2O3 extracted slag of fly ash with sodium hydroxide solution [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(6): 1928-1936.
[25] WU X, LEUNG D Y C. Optimization of biodiesel production from camelina oil using orthogonal experiment [J]. Applied Energy, 2011, 88(11): 3615-3624.
邓祥意1,冯雅丽1,李浩然2,杜竹玮2,康金星1,郭成林1
1. 北京科技大学 土木与资源工程学院,北京 100083;
2. 中国科学院过程工程研究所 生化工程国家重点实验室,北京 100190
摘 要:采用低浓度碱浸对低品位软锰矿进行预脱硅处理,考察NaOH浓度、液固比、浸出温度、浸出时间及搅拌速率对硅浸出率的影响,研究碱浸过程动力学。结果表明:在NaOH起始浓度为20%、液固比为4:1、浸出温度为180 °C、浸出时间为4 h、搅拌速率为300 r/min的条件下,硅浸出率达到91.2%。缩核模型表明,碱浸过程受化学表面反应控制,其表观反应活化能为53.31 kJ/mol。通过正交试验对脱硅渣流化焙烧制备锰酸钠的条件进行优化,在硅浸出率为91.2%、NaOH/MnO2质量比为3:1、焙烧温度为500 °C、焙烧时间为4 h的条件下,锰酸钠的转化率为89.7%,且锰酸钠转化率随硅浸出率的升高而增加。
关键词:低品位软锰矿;脱硅;流化焙烧;锰酸钠
(Edited by Wei-ping CHEN)
Foundation item: Project (2015ZX07205-003) supported by the Major Science and Technology Program for Water Pollution Control and Treatment, China; Project (DY125-15-T-08) supported by the China Ocean Mineral Resources Research & Development Program; Projects (21176026, 21176242) supported by the National Natural Science Foundation of China
Corresponding author: Ya-li FENG; Tel: +86-13910839080; E-mail: ylfeng126@126.com
DOI: 10.1016/S1003-6326(18)64742-9

