Kinetic Monte Carlo simulation of microalloying effect in Al-Ag alloys
来源期刊:中国有色金属学报(英文版)2007年第3期
论文作者:周明 李世晨 郑子樵 杨培勇
文章页码:461 - 461
Key words:Al-Ag alloy; kinetic Monte Carlo method; microalloying elements; aging stage
Abstract: The kinetic Monte Carlo method, which based on the Multi-States Ising Model, was applied to simulate the effect of microelements on the microstructural evolution of Al-Ag alloys during initial aging stage. The simulation results suggest that the microelements In, Sn and Be have a dramatic depression effect on the Ag clustering because of their strong tendency to co-existed with vacancies. There are no significant effects on the process of Ag clustering in Al-Ag alloys containing Li or Cd, because of little interaction between Li/Cd and Ag/vacancies. Microelements can influence the aging by interacting with vacancies and the atoms of precipitated composition, in which the former seems more important. In this model, “vacancy-locking” and “vacancy clusters” are two important mechanisms in the aging process.
基金信息:the National Natural Science Foundation of China

ZHOU Ming(周 明), LI Shi-chen(李世晨), ZHENG Zi-qiao(郑子樵), YANG Pei-yong(杨培勇)
School of Materials Science and Engineering, Central South University, Changsha 410083, China
Received 25 July 2006; accepted 8 December 2006
Abstract: The kinetic Monte Carlo method, which based on the Multi-States Ising Model, was applied to simulate the effect of microelements on the microstructural evolution of Al-Ag alloys during initial aging stage. The simulation results suggest that the microelements In, Sn and Be have a dramatic depression effect on the Ag clustering because of their strong tendency to co-existed with vacancies. There are no significant effects on the process of Ag clustering in Al-Ag alloys containing Li or Cd, because of little interaction between Li/Cd and Ag/vacancies. Microelements can influence the aging by interacting with vacancies and the atoms of precipitated composition, in which the former seems more important. In this model, “vacancy-locking” and “vacancy clusters” are two important mechanisms in the aging process.
Key words: Al-Ag alloy; kinetic Monte Carlo method; microalloying elements; aging stage
1 Introduction
The effect of microalloying elements on the behavior of age-hardenable alloys is an interesting physical problem addressing the mechanisms of transport and aggregation of the solute. Trace elements have been found to exert a disproportionate influence on the structure and properties of Al alloys compared with the amounts added (0.1-0.01, molar fraction). Most trace element effects arise because they modify the nucleation and/or growth characteristics of the phases which form during precipitation. This viewpoint was supported by the experiments of KIMURA and HASIGUTI[1], NUYTEN[2], NOBLE[3], OZBILEN and FLOWER[4], and MUKHOPADHYHY et al[5]. However, these researches were mostly concentrated on the qualitative investigation on the later aging stage, and there was little on the early dynamics stage in quantity. In the several minutes of the aging stage, which is the beginning and very important stage, it is difficult to find out the microstructure evolution exactly because of the limited instrument. Furthermore, there is little research at the atomic level; however, the computer simulation can describe this process unrestrictedly[6-7].
Al-Ag system alloys, which are the classical aging hardening, have more scientific interest. Al atoms and Ag atoms have similar radius size, and the G.P zones have a spherical shape and are coherent with the matrix. Thus, the effect of lattice distortion on the aging is obviated basically, and it will be propitious for comparing the simulation with the experimental result, so it is good for us to get the microalloying function essentially.
In this work, a Monte Carlo computer simulation was adopted to investigate the role of some micro- alloying elements in Al-Ag alloys.
2 Simulation model2.1 Simulation method
The computer simulations were performed on a rigid three-dimensional FCC lattice with 50×50×51 unit cells by a kinetic Monte Carlo method. Periodic boundary conditions were also adopted to eliminate the limitation of the above simulation size. As an initial microstructure of the simulation system, all the lattice sites are occupied randomly by Al, Ag and microalloying elemental (X) atoms with compositions of Al-4Ag-0.1X, (molar fraction, %). The realistic diffusion of these atoms takes place via vacancies with the concentration of about 1.5×10-4, which approximately agrees with the equilibrium vacancy concentration in pure Al at 793 K. This postulates that phase decompositions in this simulation model evolve without diminishing any quenched-in excess vacancies at simulated temperature (T) of 423 K. The exchange of randomly selected vacancy with one of its nearest neighbor atoms is allowed if the transition probability(W) is greater than a randomly generated number, x, between 0 and 1. The value of W was calculated from the symmetrical solution [8]:
W=exp(-?E/KT)/[1+exp(-?E/KT)] (1)
where ?E is the energy difference between the atomic configurations before and after the exchange, and K is the Boltzmann constant. In this paper, a Monte Carlo step(MCS) was defined as an attempt exchange of atom and vacancy. Some structural parameters characterizing the simulation microstructures were recoded as a function of Monte Carlo steps. Three statistically independent runs were performed for each alloy type for averaging purposes. A cluster was defined as a solute atom aggregate containing two or more than two solute atoms. Average size of clusters was defined as the average number of atoms inside one cluster. The probability of solute A-solute B pair is defined as
![]()
where P(AB) is the number of solute A-solute B pair, xB is the molar fraction of B atom and Z is the coordination number. Moreover, we did not consider the elastic effect induced by the difference in atomic radius among different atoms. This simplification has some influence on the morphologies of precipitates, but it is reasonable because the formation of clusters in Al alloys is generally believed to be governed by the rate of the diffusion-controlled growth rather than that of the interface-controlled growth.
2.2 Simulation parameters
The study of cluster formations using a Monte Carlo method requires an accurate estimation of interatomic interactions in alloys. In this work, the utilized pair interactions were derived from known thermodynamic or kinetics quantities using a regular solution model[9]. The utilized pair interactions for the Monte Carlo simulation are summarized in Table 1.
Table 1 Interacting parameters in Al alloys between same atom species, between different atom species, and between solute atom and vacancy, utilized in simulation (kJ/mol)
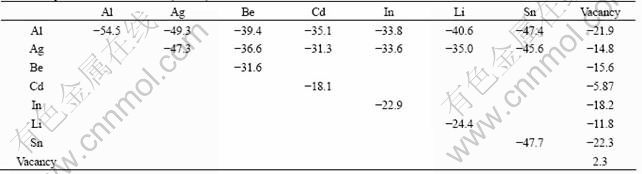
In order to compare the interaction between different atoms, the ordering parameter is defined as
Vij=εij-(εii+εjj)/2 (3)
where εii and εij are the pair interaction between the same and different atom species respectively. And the ordering parameter between atom and vacancy is defined as
VV-X=(εVX+εAlAl)-(εXAl+εAlV) (4)
3 Results and discussion3.1 Clustering of Ag atoms in Al-Ag(-X) alloy
As shown in Fig.1(a), at the beginning of the aging (solution quenching), Ag atoms distribute randomly in the Al matrix. Along with the MCS, Ag clusters form gradually. The clustering process obtained from the simulation, is consistent with the experimenting on the G.P zones[10].
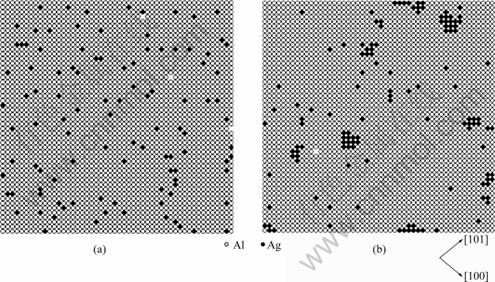
Fig.1 Typical atom configurations on one atom layer of (100) plane in Al-Ag alloys during initial stage of ageing: (a) 2MCS;(b) 8×108 MCS
Fig.2 shows the variation curves of mean size of Ag cluster (Fig.2(a)) and the residual Ag concentrations in the matrix (Fig.2(b)) in Al-Ag-X alloys during early ageing stage. The clustering process of Al-Ag-Li, Al-Ag-Cd has a similar tendency with Al-Ag binary alloy. As for the Al-Ag alloys containing other adding elements, the Ag atoms clustering was restrained strongly. According to the morphology of solute atoms and vacancies, three microalloying mechanisms were suggested.
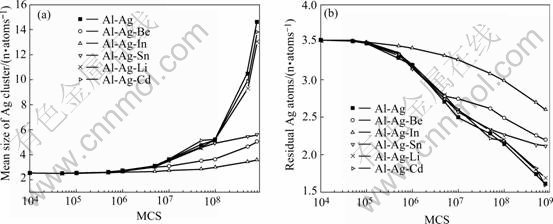
Fig.2 Variation curves of mean size of Ag cluster (a) and residual Ag concentrations in matrix (b) in Al-Ag-X alloys during early ageing stage
3.2 Influence of Li and Cd
Fig.3 shows the typical atom configurations on one atom layer of (001) plane in Al-Ag-(Li, Cd) alloys during initial stage of ageing. It could be clearly observed from Fig.3 that Ag clusters form in Al-Ag-Li and Al-Ag-Cd, which size is consistent with the results of Al-Ag shown in Fig.1(d). Li and Cd atoms distribute discretely in the Al matrix, so do vacancies. From Fig.4, it can be seen that there are not obvious crosscoupling effect between Li (Cd) and Ag, or Li (Cd) and vacancies. So the addition of Li (Cd) has no significant influence on the process of Ag clustering.
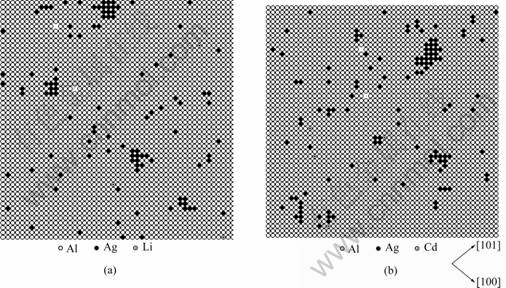
Fig.3 Typical atom configurations on one atom layer of (001) plane in Al-Ag-Li (a) and Al-Ag-Cd (b) alloys during initial stage of ageing

Fig.4 Probability of X atoms positioned adjacent to Ag atoms (a) and vacancy (b) in Al-Ag-(Li, Cd) alloys during early ageing stage
3.3 Influence of In, Sn and Be
Fig.5 shows the typical atom configurations on one atom layer of (001) plane in Al-Ag-(In, Sn, Be) alloys at MCS= 8×108. Compared with Fig.5(a), in Figs.5(b), (c)and (d) the Ag clusters with small size, the Ag-Va-In (Sn, Be) co-clusters are formed. Different from Sn and Be, the addition of In results in formation of single-vacancy type Ag-Va-In co-cluster. Fig.6 shows configuration of vacancies co-clusters in Al-Ag-(In, Sn, Be) alloys simulated for 8×108 Monte Carlo steps in 3D views. In Al-Ag-Sn or Al-Ag-Be alloy, there is only a large vacancies cluster including almost all the vacancies. However, In Al-Ag-In alloy, the small Ag-Va-In clusters with discrete distribution were observed.

Fig.5 Typical atom configurations on one atom layer of (001) plane in Al-Ag-(In, Sn, Be) alloys during initial stage of ageing
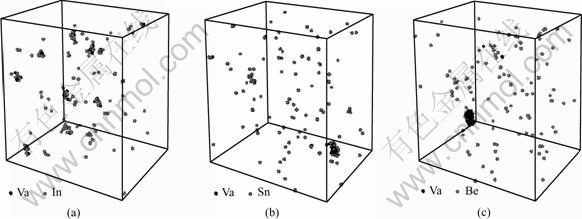
Fig.6 Configuration of clusters in Al-Ag-(In, Sn, Be) alloys simulated for 8×108 Monte Carlo steps (3D): (a) Al-Ag-In;(b) Al-Ag-Sn; (c) Al-Ag-Be
Figs.7(a) and (b) respectively show the probability of X atoms (In, Sn, Be) positioned adjacent to Ag atoms or vacancy in Al-Ag-(In, Sn, Be) alloys during early aging stage. The probability of X atoms positioned adjacent to vacancy outclasses that to Ag atoms. Before MCS=1×107, the probability of Sn, and Be positioned adjacent to Ag heightens gently, however, after then it goes up quickly. In Al-Ag-In alloy, the probability of In positioned adjacent to Ag grows up incessancy, from 4.67% in MCS=1×104 to 36.33% in MCS=8×108.
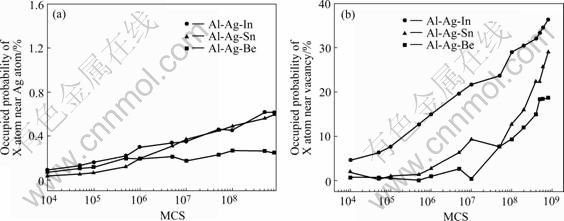
Fig.7 Probability of X atoms positioned adjacent to Ag atoms(a) and vacancy (b) in Al-Ag-(In, Sn, Be) alloys during early ageing stage
The depressed effect of Ag clustering process in Al-Ag alloys containing In, Sn and Be elements is due to the strong interaction between the microalloying elements and vacancies in alloys. The moveable vacancies decrease because of the combining of vacancy and In, Sn and Be atoms in the aging. The early aging stage of Al-Ag alloy is the typical diffusing process, so the movable vacancies results in the Ag clustering restrained. As shown in Fig.7(b), before MCS=5×106, only the near probability In atoms and vacancies is above 20% as well as others are below 6%, so none but In restrain the Ag cluster strongly. When MCS=1×107, the influence of Be appears, and later Sn does. Three of these atoms have the effect on the Ag clusters when their near probabilities with vacancies rise markedly.
In, Sn and Be elements have a suppressive effect on the Ag clustering in Al-Ag alloy, however, the mode of the effect is different. As show in Fig.6, the configuration of vacancies in Al-Ag-In alloys is far from that in the other alloys. The discrete vacancies surrounded by In atoms distribute in Al-Ag alloy. The morphology seems to reduce the movability of vacancies. And vacancies clusters with large size form in Al-Ag-Sn and Al-Ag-Be alloys, vacancies clusters play an important role on the Ag clustering. Moreover, as we know, vacancies clustering can result in vacancy collapse in practice, which is one annihilating way of in-excess vacancies.
4 Discussion
The nucleation in diffusional transformation is the atomic diffusion process. Solute atoms form clusters (embryos) first, and then grow up further, turning into nucleus of crystallization of the new phase. Therefore, the clustering evolvement in the early stage can bring pivotal influence on the precipitation.
Microalloying elements affect the clustering and aging by interplaying with the compositive elements of precipitate phase and vacancy. The major precipitate phases in Al-Ag alloy are G.P zones and γ′(Ag2Al), and Ag is the main chemical composition. Li or Cd has little crosscoupling with neither Ag or vacancy, so the addition of Li or Cd has no markedly influence on the Ag clustering. In, Sn and Be elements affect the aging by reducing the moveable vacancy.
The plot of the ordering parameters as shown in Fig.8 is very useful to predict the role of microalloying elements in Al-Ag alloy. It could be observed that Li and Cd are close with the midline, in which it is equal interaction with Ag and vacancy, and the ordering parameter VV-X and VAg-X are near zero. In, Sn and Be have a interaction with Ag equaling as Li and Cd, but they have a lower interaction with vacancy. It is well agreement with the simulation results.
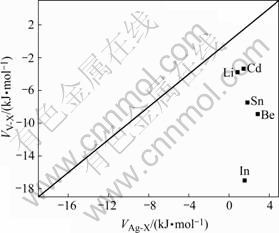
Fig.8 Relationship between ordering parameter between vacancy and X atoms(VV-X) and ordering parameter between Ag and X atoms(VAg-X) for various microalloying elemental atoms
It can be obtained from the simulation results that in the early stage of the aging in Al-Ag alloy, the trace elements In, Sn and Be have a dramatic effect on the clustering of Al-Ag alloy in the early stage, and to a lesser extent Mg does. It is well agreement with the experimental conclusion that In and Sn have a dramatic effect on the size and distribution of the phase precipitation[11]. It is also consistent with the experimental research that Sn has a retarding effect on the G.P zones formation in Al-Ag alloy[12].
The strong interaction between vacancy and In, Sn and Be atoms results in the combining of them. In Al-Ag-In alloy, the combine of In and vacancy forms Ag-Va-In clusters. It can be observed in Fig.6(a) that the vacancy is surrounded by In atoms on the whole. Figs.9(a) and (b) show the ideal atoms configuration of vacancy surrounded by In and out of In respectively. The account of ?E is ?E=E(b)-E(a)=47.58 kJ/mol>0, which shows state of Fig.9(a) is more steadier than state of Fig.9(b). So the configuration of the vacancy surrounded by In atoms is favored in energy, and the vacancy is locked in part.
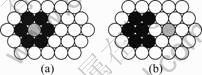
Fig.9 Ideal conditions of vacancy surrounded by In (a) and out of In (b)
In Al-Ag-Sn and Al-Ag-Be alloy, the combine of Sn and vacancy, Be and vacancy forms Ag-Va-Sn and Ag-Va-Be clusters respectively, and vacancy forms larger clusters. Figs.10(a) and (b) show the ideal atoms configuration of vacancy cluster and dissociative vacancy respectively. The account of ?E is ?E=E(b)-E(a)=50.4 kJ/mol>0, which shows state of Fig.10(a) is more steadier than state Fig.10(b). So the vacancy clusters is more steadier than single vacancies.
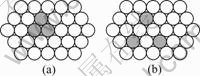
Fig.10 Ideal conditions of vacancy cluster (a) and dissociative vacancy (b)
4 Conclusions
1) Microalloying elements affect the clustering and aging by interplaying with the compositive elements of precipitate phase and vacancy.
2) The microelements In, Sn and Be have a dramatic depression effect on the Ag clustering. And the function of In chiefly is reducing the vacancy movement by locking discrete vacancy. But in Al-Ag-Sn and Al-Ag-Be alloys, forming vacancy clusters, it does not only reduces the vacancy movement, but also can result in vacancy collapsing and annihilating in practice.
3) Li and Cd have little interaction with Ag and vacancy, so they influence Ag clustering slightly.
4) The interaction between microelements and vacancy is the pivotal effect on the aging of Al-Ag alloy. “Vacancy-locking” and “vacancy clusters” are two important mechanisms in the aging process.
References
[1] KIMURA H, HASIGUTI R R. Interaction of vacancies with Sn atoms and the rate of G.P zone formation in an Al-Cu-Sn alloy [J]. Acta Metall, 1961, 9(12): 1076-1078.
[2] NUYTEN J B M. Quenched structures and precipitation in Al-Cu alloys with and without trace additions of Cd [J]. Acta Metall, 1967, 15(11): 1765-1770.
[3] NOBLE B. Theta-prime precipitation in aluminium-copper-cadmium alloys [J]. Acta Metall, 1968, 16(3): 393-401.
[4] OZBILEN S, FLOWER H M. Zirconium-vacancy binding and its influence on S′ precipitation in an Al-Cu-Mg alloy [J]. Acta Metall, 1989, 37(11): 2993-3000.
[5] MUKHOPADHYAY A, SHIFLET G J, Jr STARKE E A. Role of vacancies on the precipitation processes in Zr modified aluminum based alloys [J]. Scripta Metall Mater, 1990, 24(2): 307-312.
[6] LI Shi-chen, ZHENG Zi-qiao. Computer simulation of distribution of the solutes in Al-Cu-(Mg)-(Ag) on initial aging stages [J]. Journal of Central South University of Technology: Natural Science, 2000, 31(5): 441-444. (in Chinese)
[7] LI Shi-chen, ZHENG Zi-qiao, LIU Zu-yan, LI Jian. Monte Carlo study of the microstructural evolution of Al-Cu-(XMg) alloys during initial aging stage [J]. The Chinese Journal of Nonferrous Metals, 2005, 15(9): 1376-1382. (in Chinese)
[8] RABBE D. Computational Materials Science: The Simulation of Materials Microstructure and Properties [M]. Weiheim: Wiley, VCH, 1998.
[9] HIROSAWA S, SATO T, KAMIO A, FLOWER H M. Classification of the role of microalloying elements in phase decomposition of Al based alloys [J]. Acta Mater, 2000, 48: 1797-1806.
[10] DUBEY PH A, SCHONFELD B, KOSTORZ G. Shape and internal structure of Grinier-Preston zones in Al-Ag [J]. Acta Metall Mater, 1991, 39(6): 1161-1170.
[11] PRABHU N, HOWE J M. The effect of ternary trace additions on the nucleation and growth of γ′ precipitates in an Al-4.2at.pct Ag alloy [J]. Metallurgical Transactions A, 1992, 23: 135-148.
[12] KADI-HANIFI M, YOUSFI H, TOUATI A. Influence of Zn and Sn on the GP zones formation and the γ′ metastable phase precipitation in Al-Ag-Sn(Zn) alloys [J]. Materials Science Forum, 2002, 396/402: 995-998.
Foundation item: Project(50271084) supported by the National Natural Science Foundation of China
Corresponding author: LI Shi-chen; Tel/Fax: +86-731-8877227; E-mail: s-maloy@mail.csu.edu.cn

