改性壳聚糖对湘江河水中重金属污染物Cr(VI)的吸附
来源期刊:中国有色金属学报(英文版)2013年第10期
论文作者:刘韵琴 刘云国 胡新将 郭一明
文章页码:3095 - 3103
关键词:改性壳聚糖;印迹;重金属污染物;Cr(VI);吸附;去除率;动力学模型
Key words:modified chitosan; imprinting; heavy metal pollutants; Cr(VI); adsorption; removal rate; kinetic model
摘 要:利用分子印迹技术和甲基丙烯酸对壳聚糖进行改性,并在改变吸附条件、吸附动力学和吸附等温线的基础上,对湘江样水中Cr(VI)进行吸附研究。结果表明:X射线衍射谱显示印迹聚合物的结晶能力减弱,但非结晶区面积增加,吸附点位数提高,对Cr(VI)的吸附容量增大;印迹聚合物对Cr(VI)的吸附能力随时间的延长而增加,8 h后达到饱和,最佳吸附时间是吸附后4~8 h,对Cr(VI)的提取率最大值为33.7%。提取液最佳pH 值是4.5~7.5;提取率随着壳聚糖脱乙酰度的增大而增大,吸附效果最好的是90%脱乙酰度壳聚糖。吸附量随着壳聚糖的浓度增加而增加,饱和后对Cr(VI)的提取率变化相对平稳,实验测得最高去除率为98.3%。Cr(VI)印迹壳聚糖吸附的准一级动力学和二级动力学模型线性相关系数分别是0.9013和0.9875,吸附速率分别为0.0091 min-1和7.129 g/(mg·min)。Cr(VI)印迹壳聚糖的吸附更符合二级动力学模型,与Langmuir 吸附等温线的拟合性比 Freundlich吸附等温线的更好,计算得到的最大吸附容量为 15.784 mg/g,对河水中Cr(VI)的吸附效果明显。
Abstract: Methacrylic acid was used together with a molecular imprinting technique to modify chitosan. In addition, the adsorption kinetics and adsorption isotherms were recorded and the results were analyzed to investigate reparative adsorption for Cr(VI) from the polluted Xiangjiang River water. A comparative X-ray analysis shows that the degree of crystallization in the imprinted polymer was significantly weakened, the area of the non-crystalline region was larger. There were more adsorption sites in the imprinted polymer, and the adsorption capacity towards Cr(VI) was increased. The adsorption capacity of the imprinted polymer towards Cr(VI) increased with time and reaches saturation after 8 h. The optimal adsorption time was 4-8 h after the adsorption starting and the optimal pH value for the solution was in the range of 4.5-7.5. When the chitosan reaches saturation, the adsorption capacity achieves a state of equilibrium, and the maximum Cr(VI) extraction rate reaches 33.7%. Moreover, the adsorption capacity of the imprinted polymer towards Cr(VI) increases with increasing chitosan concentration. In this situation, the Cr(VI) extraction rate shows little variation, and the maximum removal rate can reach 98.3%. Furthermore, the Cr(VI) extraction rate increases with an increase in the degree of deacetylation in the chatoyant and chitosan, with the best adsorption effect corresponding to 90% deacetylation. Fitting the adsorption data to the quasi first- and second-order kinetic models yields correlation coefficients of 0.9013 and 0.9875, respectively. The corresponding rate constants for the two models are 0.0091 min-1 and 7.129 g/(mg·min), respectively. Hence, the adsorption using Cr(VI)-imprinted chitosan is more consistent with the second-order kinetics. Comparing the data to Freundlich and Langmuir adsorption isotherms shows that the latter has a better linear fit and a maximum adsorption capacity of 15.784 mg/g.
Trans. Nonferrous Met. Soc. China 23(2013) 3095-3103
Yun-qin LIU1,2, Yun-guo LIU1, Xin-jiang HU1, Yi-ming GUO1
1. College of Environmental Science and Engineering, Hunan University, Changsha 410082, China;
2. Department of Humanity & Tourism, Hunan Vocational College of Commerce, Changsha 410205, China
Received 29 September 2013; accepted 8 October 2013
Abstract: Methacrylic acid was used together with a molecular imprinting technique to modify chitosan. In addition, the adsorption kinetics and adsorption isotherms were recorded and the results were analyzed to investigate reparative adsorption for Cr(VI) from the polluted Xiangjiang River water. A comparative X-ray analysis shows that the degree of crystallization in the imprinted polymer was significantly weakened, the area of the non-crystalline region was larger. There were more adsorption sites in the imprinted polymer, and the adsorption capacity towards Cr(VI) was increased. The adsorption capacity of the imprinted polymer towards Cr(VI) increased with time and reaches saturation after 8 h. The optimal adsorption time was 4-8 h after the adsorption starting and the optimal pH value for the solution was in the range of 4.5-7.5. When the chitosan reaches saturation, the adsorption capacity achieves a state of equilibrium, and the maximum Cr(VI) extraction rate reaches 33.7%. Moreover, the adsorption capacity of the imprinted polymer towards Cr(VI) increases with increasing chitosan concentration. In this situation, the Cr(VI) extraction rate shows little variation, and the maximum removal rate can reach 98.3%. Furthermore, the Cr(VI) extraction rate increases with an increase in the degree of deacetylation in the chatoyant and chitosan, with the best adsorption effect corresponding to 90% deacetylation. Fitting the adsorption data to the quasi first- and second-order kinetic models yields correlation coefficients of 0.9013 and 0.9875, respectively. The corresponding rate constants for the two models are 0.0091 min-1 and 7.129 g/(mg·min), respectively. Hence, the adsorption using Cr(VI)-imprinted chitosan is more consistent with the second-order kinetics. Comparing the data to Freundlich and Langmuir adsorption isotherms shows that the latter has a better linear fit and a maximum adsorption capacity of 15.784 mg/g.
Key words: modified chitosan; imprinting; heavy metal pollutants; Cr(VI); adsorption; removal rate; kinetic model
1 Introduction
Chitosan’s chemical name is (1,4)-2-amino-2- deoxy-β-D-glucose and it is a linear polysaccharide. Due to its poor water solubility and mechanical properties, its application is limited to some degree. However, there are numerous free amino groups in its molecular chain. There are also hydroxyl groups at the ortho-position of the amino groups whose hydrogen bonding can easily be ‘borrowed’. Thus, many types of chemical modification can be undertaken to produce a series of chitosan derivatives. This can improve chitosan’s water solubility, biological activity, and mechanical properties, which can facilitate its application in various fields [1-3]. Ionic bonding can also be utilized to form the molecule with a reticular structure so that it can stably coordinate to metal ions. Therefore, it can be used as a collector of heavy metal ions.
As early as 1980s, CATHERINE [4] investigated chitosan’s ability to adsorb Pb2+ and Cr(VI). Its adsorption capacity was found to reach 0.2 and 0.25 mg/g, respectively. HAIDER and PARK [5] estimated that the maximum adsorption capacities towards these elements could reach up to 485.4 and 263.2 mg/g, respectively. Taking glutaraldehyde as the crosslinking agent, BARONIA et al [6] prepared a modified chitosan membrane and investigated its adsorption ability with respect to Cr(VI). GRACIELA et al [7] used the Schiff reaction to conduct crosslinking of chitosan and glutaraldehyde. As a result, they obtained a flaked product (0.85-2.0 mm in size). When the pH value was 4.0, the adsorption capacity towards Cr(VI) reached 215 mg/g after 96 h. RAJENDRA et al [8] and BINGJIE et al [9] pretreated chitosan for adsorption of lead (II) from water and studied the adsorption properties of Cd(II)-imprinted chitosan resin separately and their results show efficiency.
Chromium (Cr) oxidation is widely used in many industries such as those associated with electroplating, leather processing, ore washing, printing, dyeing. All chromium compounds are toxic but the most toxic oxidation state is Cr(VI). Taking the Xiangjiang River, China as an example, about 3.94 t highly toxic heavy metal chromium compounds and 2.8×106 t Cr(VI)- containing industrial wastewater are directly discharged into the river each year [10]. Among the pollutants related to Cr, 84.3% are concentrated in the Chang-Zhu- Tan reach of the Xiangjiang River. This tremendously polluted feels the full impact of the heavy metal Cr(VI). Reports show that the average chromium compound pollution, especially Cr(VI) pollution, exceeds the safety standard by 4.6-13.22 times [10]. This high pollution level has great destructive power towards animals, plants, the general ecological environment in the vicinity of the river water bank, and the drainage basin.
Using a molecular imprinting technique, this work aims at modifying chitosan so that it will efficiently react with Cr(VI) as a template. A polyethylene glycol (PEG) 400 macromonomer with a methacrylic acid (MAA) end group was employed in the reaction. In this way, the host-guest coordination compounds and crosslinking agents can copolymerize around the template molecule via free radicals to form a rigid polymer with high crosslinking. After pickling, the polymer obtained was transformed into an adsorption-imprinted polymer material. By changing the adsorption conditions for Cr(VI) such as the adsorption temperature and the dosage of imprinted polymer, the effect of chitosan modification on the adsorption of Cr(VI) in the river water samples was investigated. Meanwhile, the adsorption kinetics and isotherms were also analyzed to research the absorbing capacity of the imprinted polymer, therefore to repair the Cr(VI) polluted water in Chang-Zhu-Tan reach of the Xiangjiang River.
2 Experimental
2.1 Sample water
Sample water was taken from 12 check points in the Chang-Zhu-Tan area of the Xiangjiang River as demonstrated in Fig. 1. An improved form of diphenylcarbazide spectrophotometry was adopted to measure and test the content of Cr(VI) in the sample water. The degrees of pollution due to the heavy metal Cr(VI) are different at various check points in Table 1. And the Cr(VI) concentration of all the sample water was 5.21 mg/L (standard error: 0.5 mg/L), which was obtained and used for the main average experiments.
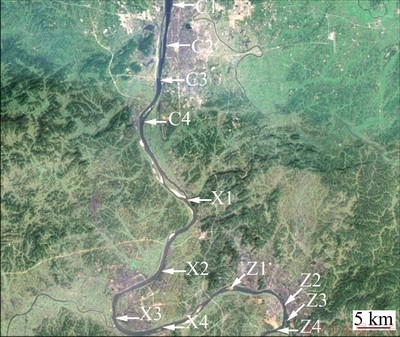
Fig. 1 Sampling points in Chang-Zhu-Tan reach of Xiangjiang River used for collection of river water polluted by Cr(VI)
2.2 Reagents and instrumentation
The main materials were chitosan (80%-95% DOD), MAA, polyethylene glycol (PEG) 400 macromonomer with one end group and two end groups, and analytical reagents such as water-soluble sodium chromate (Na2Cr2O7) and potassium dichromate (K2Cr2O7).
The main equipments used included magnetic stirrers (GSP-77-03), an X-ray diffractometer (Rigaku D/MAX 2200VPC), pH meter (PHS-3C, Shanghai Yulong Instrument Co., Ltd.), diphenyl carbazide spectrophotometer for detection of Cr(VI), and atomic absorption spectrophotometer (KLAS-1000CA).
2.3 Methods
2.3.1 Preparation of imprinted chitosan polymer
The PEG 400 macromonomer with one end group and that with two end groups and 1172 photoinitiator (4% by mass of the total mass of the two macromonomers), respectively, were added into a chitosan solution (2.5%). The solution obtained was stirred uniformly. Certain amounts of chitosan and MAA were added to the solution to adjust its viscosity. Then, 1%-5% of the total mass of chitosan and PEG 400 macromonomer with one end group, water-soluble sodium and potassium dichromates containing Cr(VI) were added into the solution to conduct imprinting.
Table 1 Cr(VI) concentrations at 12 sampling points in Fig. 1

The imprinted solution was placed under a UV lamp to facilitate polymerization. Aqueous ammonia was subsequently added to adjust the pH value of the solution to 6.5 and no further pH change was present at 25 °C. During this process, the amino groups in the chitosan were ionized, and at the same time, the MAA was transformed into the corresponding methacrylamide (MAM). After this, the product was filtered, washed, and pickled until there was no Cr(VI) present in the washing liquor. Washing was continued until a neutral solution was obtained and the product was dried to constant mass in vacuum. As a result, a polymeric adsorbent with Cr(VI) holes was obtained. In this product, the mass ratio of chitosan to PEG 400 macromonomer (with one MAA end group) was 2:3.
2.3.2 Measurement of adsorption rate of imprinted chitosan towards Cr(VI)
The extraction rate of Cr(VI) due to adsorption by imprinted chitosan was calculated according to the following formula:
G=10-3×CNV/m×100% (1)
where G denotes the extraction rate from the sample water of Cr(VI) by the chitosan, C represents the final mass concentration detected in the sample water, N refers to the dilution multiple of the initial extracting solution, and V denotes the volume of the initial extracting sample water. The quantity m represents the Cr(VI) mass in each gram of sample water.
2.3.3 X-ray characterization of imprinted polymer
Certain amounts of chitosan, imprinted-polymer (that is, the polymer without the Cr(VI) template), and imprinted polymer were taken and subjected to X-ray analysis (Rigaku D/MAX 2200VPC X-ray diffracto- meter). In the detection process, the working voltage was 50 kV, while the working current was 30 mA. Moreover, the Cu Kα line of Ni filtering was utilized, and the diffraction angle 2θ was 5°-55°.
2.3.4 Adsorption of heavy metal pollutant Cr(VI) by imprinted polymer
To test the Chang-Zhu-Tan reach sample water (with an average Cr(VI) content of 5.21 mg/L), a definite amount of Cr(VI) was taken and mixed with the imprinted polymer. By changing the experimental conditions, the effects of factors, such as the amount of the imprinted polymer, the adsorption temperature, the adsorption time could be investigated. Meanwhile, an atomic absorption spectrophotometer (KLAS-1000CA) was used to test the Cr(VI) content in the adsorption solutions obtained under different conditions. Then, the adsorption efficiency of the imprinted polymer was determined.
3 Results and discussion
3.1 Influence of Cr(VI) imprinting on crystal structure of polymer
The partial aim of this work is to fully understand the capability of the imprinted chitosan to repair river water polluted by the heavy metal Cr(VI). To achieve this aim, crystallization in the degraded chitosan, non-imprinted chitosan, and Cr(VI)-imprinted chitosan was investigated at different diffraction angles (2θ= 5°-55°). The relevant X-ray diffraction (XRD) patterns for the three types of chitosan are shown in Fig. 2.
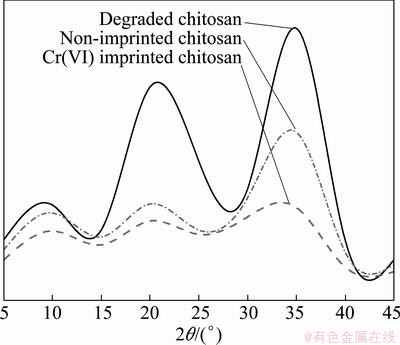
Fig. 2 XRD patterns from three chitosan polymerization forms in 2θ range of 5°-55°
As can be seen from Fig. 2, degraded chitosan presents three diffraction peaks at 2θ of 9.5°, 13.9° and 21.3°. For non-imprinted chitosan, the diffraction peak at 13.9° is not present, while that at 21.3° is significantly weakened. Thus, the amorphous adsorption area has become large. This indicates that the molecular chain regularity in the chitosan is destroyed in the non-imprinted polymerization process and the molecular activity is thereby restrained. Consequently, the degree of crystallization in the imprinted polymer is reduced. Compared with non-imprinted chitosan, Cr(VI)- imprinted chitosan presents a much lower relative intensity in the diffraction peak at 21.3°, and crystallization degree is further reduced. Thus, it can be inferred that in the imprinted polymerization process (i.e. imprinting chitosan with Cr(VI) as the template), the coordination and adsorption of Cr(VI) not only occurs in the non-crystalline region of the polymer, but also penetrates into the crystalline region of the polymer. At the same time, this destroys the coordination of Cr(VI) in the crystalline region to the amino and hydroxyl groups on the molecular chain of the chitosan. On this basis, the hydrogen bonding in the molecular chain, or among molecular chains, is thereby destroyed. The coordination bonds formed in this way show a certain crosslinking action. As a result, the crystallization capacity of the imprinted polymer is weakened [6,9,11]. The weakened crystallization capacity of the imprinted polymer leads to an increase in the area of the non-crystalline region, the number of adsorption sites and the capacity to adsorb Cr(VI).
3.2 Adsorption effect of imprinted polymer towards Cr(VI)
3.2.1 Influence of Cr(VI) concentration on adsorption capacity of imprinted chitosan
Imprinted chitosan was dissolved and prepared into chelating agents with different concentrations. A sample (20 mL) of each chelating agent was adjusted to a certain pH value and used to treat water with a known Cr(VI) concentration. The adsorption rate was then calculated. The solution without chitosan was used as a reference. When the pH value is 3, the effect of Cr(VI) concentration on the extraction rate follows the pattern exhibited in Fig. 3. As demonstrated in Fig. 3, the Cr(VI) extracted from the experimental sample water increases with increasing Cr(VI) concentration. When the Cr(VI) concentration is in the range of 0.1-0.3 mg/L, the extraction rate increases rapidly with the increase in concentration. When the concentration is 0.4 mg/L, the extraction rate becomes quite stationary. As the Cr(VI) concentration continues to increase, there is little variation in the Cr(VI) extraction rate and the maximum extraction rate of Cr(VI) reaches 31.7%. So, it can be inferred that over the current experimental concentration range, the adsorption capacity of chitosan for Cr(VI) increases with rising concentration (Fig. 3).

Fig. 3 Influence of Cr(VI) concentration on adsorption capacity of imprinted chitosan
3.2.2 Effect of chitosan DOD on adsorption
Chitosan with 80%, 85%, 90%, and 95% DOD was dissolved and made into extracting solutions with a concentration of 0.5 mg/L. Following the same method used above, the extraction rate of Cr(VI) was then calculated. The data show that as the DOD of the chitosan gradually increases from 80% to 95%, the Cr(VI) extraction rate from the experimental sample water also increases. This is because the larger the DOD of the chitosan, the greater the number of free amino groups on the chitosan skeleton and thus the greater the likelihood that the chitosan will chelate Cr(VI). As a result, the Cr(VI) extraction rate increases. However, chitosan with a DOD higher than 90% presents no further significant increase in adsorption. In addition, taking economic factors into account, chitosan with a DOD of 90% is the best choice to repair wastewater, as it has good solubility and it is not too expensive. Comparatively, chitosan with a DOD lower than 85% is difficult to dissolve, while chitosan with a DOD of 95% is easily expanded but very expensive.
3.2.3 Effect of absorbent dosage of pollutant Cr(VI) on chitosan’s extraction ability
Polluted water samples with different Cr(VI) contents were taken and used for adsorption experiments. The adsorption time was set as 8 h, while the pH values of the solutions were around 6. The initial concentration of Cr(VI) was 30 mg/L and the adsorption was conducted at 25 °C. Under these conditions, the Cr(VI) extraction rates adsorbed by the chitosan in the water samples with different exogenous Cr(VI) contents were calculated. When the contents of Cr(VI) in the sample water were set as 68.3%, 73.2%, 87.4%, 90.7%, 93.1%, 95.3%, and 98.1%, the corresponding adsorbent dosages of the imprinted chitosan were 0.05, 0.1, 0.15, 0.2, 0.25, 0.3, and 0.35 g, respectively. After the solution was shaken for 1 h, it was filtered and the supernatant liquid was analyzed to find the residual Cr(VI) concentration and used to calculate the removal rate of the Cr(VI). The influence of the adsorbent dosage on the removal rate of Cr(VI) is displayed in Fig. 4. The experimental results indicate that as the adsorbent dosage becomes large, the removal rate of Cr(VI) in the solution also increases. When the dosage is 0.4 mg/L, the removal rate of Cr(VI) reaches 98.3%. Hence, it can be deduced reversely that when the chitosan concentration, the pH value, temperature, and adsorption time are kept constant, the extraction rate of Cr(VI) by the chitosan gradually declines as the Cr(VI) content in the water sample increases.
3.2.4 Effect of extraction time on adsorption rate
Chitosan with a concentration of 0.4 mg/L was used to treat sample water with a Cr(VI) content of 5 mg/L at 25 °C. During the adsorption process, the pH value of the solution approaches 7. The Cr(VI) extraction rate was tested at different adsorption time, as demonstrated in Fig. 5(a). As shown in Fig. 5(b), the chitosan’s extraction rate of Cr(VI) from the sample water gradually increases with time. However, when the adsorption duration is longer than 8 h, the chitosan’s adsorption capacity reaches saturation, that is, the adsorption process stops. In this situation, the extraction rate of Cr(VI) achieves the maximum value of 31.6%.
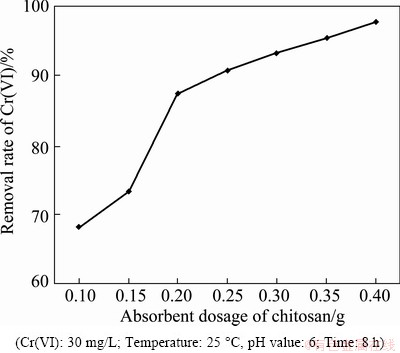
Fig. 4 Influence of absorbent dosage on chitosan’s adsorption rate

Fig. 5 Effect of adsorption time (a) and pH value (b) on Cr(VI) adsorption rate from sample water using imprinted chitosan
3.2.5 Effect of pH on Cr(VI) extraction
In this experiment, chitosan with a concentration of 0.4 g/L was used to treat sample water with a Cr(VI) content of 7.22 mg/L. The extraction time used was 10 h and the temperature was 25°C. The chitosan’s extraction rate of Cr(VI) from the sample water was tested at different pH values (Fig. 5(b)). The pH value has a significant influence on the adsorbent’s ability to adsorb Cr(VI). When the pH value increases from 2.0 to 6.0, the Cr(VI) extraction rate gradually rises. When the pH value is 6, the maximum extraction rate is reached. But when the pH value is larger than 6, the Cr(VI) extraction rate gradually decreases. LIU et al [9] proved that in acid solution, the protonated amino groups in the chitosan and the anions 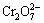 or
or 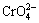 of Cr(VI) are bonded by electrostatic interactions and hydrogen bonding between the two. In other words, in the sample water, Cr(VI) exists in various forms such as
of Cr(VI) are bonded by electrostatic interactions and hydrogen bonding between the two. In other words, in the sample water, Cr(VI) exists in various forms such as 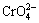 ,
, 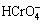 and
and 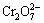 , and their relative proportions are determined by the concentration of Cr(VI) and the pH value of the solution. In the alkaline or neutral solution, Cr(VI) mainly takes the form of
, and their relative proportions are determined by the concentration of Cr(VI) and the pH value of the solution. In the alkaline or neutral solution, Cr(VI) mainly takes the form of 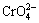 , while in acid solution of pH value ranging from 2 to 6,
, while in acid solution of pH value ranging from 2 to 6, 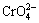 and
and 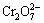 are in equilibrium. With decreasing pH value, the equilibrium moves towards
are in equilibrium. With decreasing pH value, the equilibrium moves towards 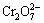 . However, when the Cr(VI) concentration is low,
. However, when the Cr(VI) concentration is low, 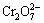 exists principally in the form of
exists principally in the form of 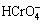 [12].
[12].
During the interaction between protonated —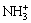 in the chitosan and Cr(VI), the protonated —
in the chitosan and Cr(VI), the protonated —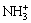 can adsorb one Cr(VI) when interacting with
can adsorb one Cr(VI) when interacting with 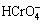 . For the same adsorption site, the protonated —
. For the same adsorption site, the protonated —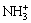 can only adsorb 50% of Cr(VI), equivalently, when interacting with
can only adsorb 50% of Cr(VI), equivalently, when interacting with 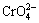 . So, the adsorption process may be theoretically ideal, but for the practical reparative regulation of river water polluted by Cr(VI), there is a poor or even insignificant adsorption effect. It can be inferred that chitosan in acidic solution presents a higher adsorption efficiency and larger adsorption capacity. However, there is a notable problem. When the acidity of the solution is excessively high, chitosan shows significant acid-solubility in the solution [9]. Consequently, the adsorption effect is greatly weakened. Overall, excessively high acidity is harmful to adsorption. The experimental results indicate that the most suitable pH value to use for the chitosan extracting solution is in the range from 4.5 to 7.5, as shown in Fig. 5(b).
. So, the adsorption process may be theoretically ideal, but for the practical reparative regulation of river water polluted by Cr(VI), there is a poor or even insignificant adsorption effect. It can be inferred that chitosan in acidic solution presents a higher adsorption efficiency and larger adsorption capacity. However, there is a notable problem. When the acidity of the solution is excessively high, chitosan shows significant acid-solubility in the solution [9]. Consequently, the adsorption effect is greatly weakened. Overall, excessively high acidity is harmful to adsorption. The experimental results indicate that the most suitable pH value to use for the chitosan extracting solution is in the range from 4.5 to 7.5, as shown in Fig. 5(b).
3.3 Adsorption kinetics of imprinted polymer for Cr(VI)
The adsorption kinetics is a useful measure characterizing adsorption rate [13]. As illustrated in Fig. 5(a), due to the superiority of the imprinted modified chitosan material, after 0.5 h, the Cr(VI) in the solution is rapidly adsorbed onto the adsorbent. After 2 h, the solution is in the adsorption plateau phase, while 4 h later, the adsorption equilibrium is mainly achieved. It is also found during the test process that the adsorbent surface becomes wet and swollen. This accelerates the diffusion rate into the membrane and holes. In this way, the adsorption rate is thereby improved. Therefore, two kinetic models were adopted to investigate the adsorption rate of the imprinted polymer towards Cr(VI) in the water sample.
3.3.1 Pseudo-first-order kinetic model
To account for the absorption, we first attempt to fit the data to a pseudo-first-order kinetic model. The equation for pseudo-first-order kinetics can be expressed as [14-17]
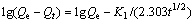 (2)
(2)
where K1 denotes the adsorption rate constant, Qt represents the adsorption capacity at time t and Qe represents the adsorption capacity at equilibrium. The constant K1 can be found by a linear fitting to a plot of lg(Qe-Qt) versus t.
As mentioned above, the water samples were taken from 12 points along the Chang-Zhu-Tan reach of the Xiangjiang River. So, the actual pollution level, and the influencing factors at each point will be different. Even if the water samples are uniformly mixed, the adsorption of Cr(VI) by the imprinted chitosan cannot be completely consistent with the first-order kinetics model. The adsorption rate of this model approaches 0.0091 min-1 (Fig. 6).
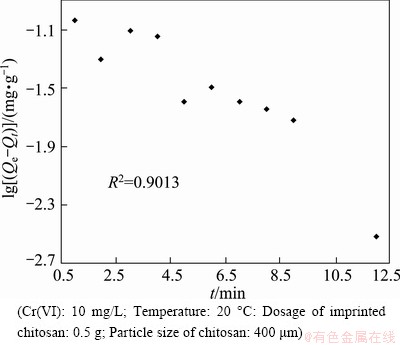
Fig. 6 Plotting data to fit pseudo-first-order kinetics model
3.3.2 Pseudo-second-order kinetic model
The pseudo-second-order kinetics model (Fig. 7) was found to give a better fit to the Cr(VI) adsorption data. The equation for pseudo-second-order kinetics can be expressed as [18]
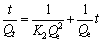 (3)
(3)
where K2 denotes the adsorption kinetic rate constant, Qt represents the adsorption capacity at time t and Qe refers to the adsorption capacity at equilibrium. Thus, the adsorption constant K2 can be acquired by plotting and fitting to a curve with t/Qt as the ordinate and t as the abscissa. The greater linearity of Fig. 7 suggests that the adsorption of Cr(VI) by the imprinted chitosan is more consistent with the pseudo-second-order kinetics. Further, the adsorption kinetic rate constant approaches to 7.129 g/( mg·min).
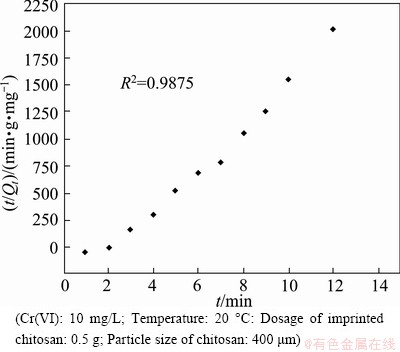
Fig. 7 Plotting data to pseudo-second-order kinetics model
Based on the data in Figs. 6 and 7, the linear correlation coefficients for fitting the pseudo-first- order and pseudo-second-order models can be calculated: both are larger than 0.9. Thus, the correlation is good. Therefore, from the point of view of the adsorption kinetics, the kinetics of Cr(VI) adsorption using the imprinted polymer is simultaneously in agreement with both the pseudo-first-order and pseudo-second-order models. However, the pseudo-second-order model shows a better correlation coefficient (0.9875).
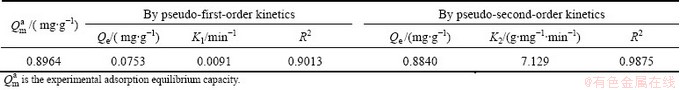
The results obtained by data fitting are shown in Table 2. As shown in Table 2, the adsorption equilibrium capacity derived using the pseudo-first-order kinetic model is 0.0753 mg/g, which is very different from the experimental adsorption equilibrium capacity (0.8211 mg/g compared with 0.8964 mg/g). The adsorption equilibrium capacity found using the pseudo-second- order kinetic model is 0.8848 mg/g, which is only 0.0116 mg/g different from the experimental value. This further confirms that the adsorption of Cr(VI) by imprinted chitosan is far more consistent with the pseudo-second- order kinetic model.
Table 2 Parameters derived for adsorption kinetics
3.4 Adsorption isotherms
Adsorption isotherms for metal ions are generally expressed using the Langmuir or Freundlich models. The Langmuir equation used here takes the following form [18-20]:
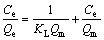 (4)
(4)
where Ce denotes the equilibrium concentration of the heavy metal solution, Qe is the adsorption capacity at equilibrium, KL is the adsorption partition coefficient, and Qm refers to the maximum adsorption capacity.
The Freundlich isothermal adsorption equation can be written as
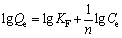 (5)
(5)
where KF denotes the maximum adsorption capacity and n is a constant related to adsorption intensity.
As illustrated in Figs. 3 and 4, the Cr(VI) adsorption capacity of imprinted chitosan increases with increasing concentration of Cr(VI). Figures 8 and 9 show the data fitting to the Langmuir and Freundlich isothermal adsorption equations. A comparison of the linear correlation coefficients in the two figures indicates that the Langmuir adsorption isotherm presents a better fit compared with the Freundlich model. The maximum adsorption capacity calculated from the Langmuir model is 15.784 mg/g, as demonstrated in Figs. 8 and 9.
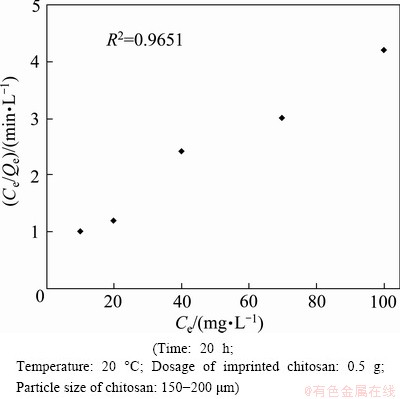
Fig. 8 Fitting data to Langmuir isotherm

Fig. 9 Fitting data to Freundlich isotherm
3.5 Adsorptive selectivity and repeated use performance
The performance of an imprinted polymer for selecting the template material is commonly characterized by the static partition coefficient KD and the separation factor α, which are defined by the following formulas:
 (6)
(6)
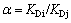 (7)
(7)
where Q denotes the adsorption capacity of the material on the polymer, CR represents the material’s equilibrium concentration in the solution, and i and j refer to the template molecule and the substrate, respectively.
As the samples used in this research consist of river water polluted by Cr(VI), the situation in this work is different from that often encountered under the absolute conditions used in the laboratory. The water in the Xiangjiang river in the Chang-Zhu-Tan area has many other heavy metal pollutants whose properties and structures are similar to those of the template material. Thus, competitive adsorption is likely to be generated between these pollutants and the template material, and this will affect the adsorption efficiency. Hence, the adsorption of manganese (Mn), cadmium (Cd), and iron (Fe) were investigated to reveal their effect on Cr(VI) adsorption by the imprinted polymer. These metals were chosen as they are in the same region of the periodic table or show the same valency as Cr. The results are displayed in Fig. 10. It demonstrates that the separation factors of Cr(VI)/Cd(II), Cr(VI)/Fe(III), and Cr(VI)/ Mn(II) are 1.40, 2.5, and 3.45, respectively. Thus, they are all larger than 1.35. This indicates that the imprinted polymer can separate the template material from the mixture and thus shows good selectivity [9].
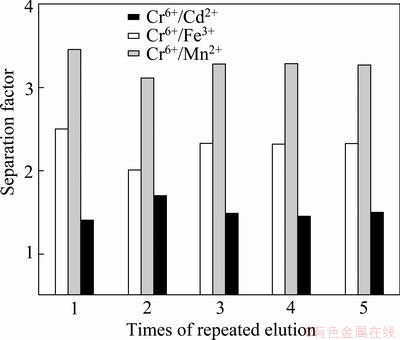
Fig. 10 Selectivity and repeated use performance of imprinted polymer
Keeping other conditions constant, the imprinted polymer without the template and chitosan were used to conduct a comparison experiment. The experimental results show that the separation factors were all around 1. This indicates that the imprinted polymer without the template and chitosan show no selective adsorption capacity towards Cr(VI).
Figure 10 shows the effect of 4 cycles of adsorption and elution. Among Cr(VI)/Cd(II), Cr(VI)/Fe(III), and Cr(VI)/Mn(II) separation factors, each tends to become stable over the cycles. Thus, it can be inferred that the adsorption capacity and selectivity of the imprinted polymer are not impaired after many cycles of adsorption/elution. Therefore, the imprinted polymer prepared in the experiment has good regenerability, stable adsorption levels, and performs well after repeated use.
4 Conclusions
1) The XRD results indicate that the degree of crystallization of the imprinted polymer is reduced, which leads to an increase in both the area of the non-crystalline region and the number of adsorption sites in the chitosan. Thereby, the adsorption capacity with respect to Cr(VI) is improved.
2) When the pH value is 6.5 and the temperature is 25 °C, the adsorption capacity of chitosan towards Cr(VI) increases with increasing chitosan concentration. When the concentration is 0.4 mg/L, a stationary value of the extraction rate is achieved, and its maximum value is 33.7%.
3) The adsorption capacity of imprinted chitosan towards Cr(VI) increases with time. After 8 h, the saturated state begins to be produced. The optimal adsorption time is 4-8 h. In addition, the optimal pH value of the chitosan extracting solution is 4.5-7.5. Furthermore, the Cr(VI) extraction rate increases with an increase in the degree of deacetylation in the chatoyant and chitosan, with the best adsorption effect corresponding to 90% deacetylation. In this work, the maximum removal rate of Cr(VI) eliminated by imprinted chitosan reaches 98.3%.
4) The linear correlation coefficients from data fitting to the pseudo-first-order and pseudo-second-order kinetic models are 0.9013 and 0.9875, respectively. The corresponding rate constants for adsorption are 0.0091 min-1 and 7.129 g/(mg·min). Thus, Cr(VI) adsorption by the imprinted chitosan is more consistent with the second-order model. In addition, the Langmuir adsorption isotherm shows a better linear fit compared with the Freundlich model. The maximum adsorption capacity reaches 15.784 mg/g.
References
[1] WANG Xia. Study on preparation and biological activities of precolum derivatization chitosan [D]. Wuhan: Central China Normal University, 2008. (in Chinese)
[2] ALIREZA A, MOHAMMED A. Applications of chitosan in the seafood industry and aquaculture: A review [J]. Food Bioprocess Technol, 2012, 5: 817-830.
[3] LIPATOVA I M, MEEZINE E A. Synthesis of chitosan-mineral sorbents on fibrous supports and study of their properties [J]. Russian Journal of Applied Chemistry, 2012, 7: 1059-1063.
[4] CATHERINE A E. Chitosan [J]. Journal of Applied Polymer Science, 1980, 25: 1587-1599.
[5] HAIDER S, PARK S Y. Preparation of the electrospun chitosan nanofibers and their applications to the adsorption of Cu(II) and Pb(II) ions from an aqueous solution [J]. Journal of Membrane Science, 2009, 328: 90-96.
[6] BARONIA P, VIEIRAA R S, MENEGHETTIA E, DASILVAA M G C, BEPPU M M. Evaluation of batchadsorption of chromium ions on natural and crosslinked chitosan membranes [J]. Journal of Hazardous Materials, 2008, 152: 1155-1163.
[7] GRACIELA R, JORGE S, JAIME A F. Adsorption of chromium onto cross-linked chitosan [J]. Separation and Purification Technology, 2005, 44(1): 31-36.
[8] RAJENDRA D, MINAKSHI T, DINESH G, JOSTNA M B. Pretreated chitosan for adsorption of lead(II) from water [J]. Bulletin of Materials Science, 2012, 35: 875-884.
[9] LIU Bing-jie, WANG Dong-feng, XU Ying, HUANG Guo-qing. Adsorption properties of Cd(II)-imprinted chitosan resin [J]. Journal of Materials Science, 2011, 46: 1535-1541.
[10] LIU Yun-qin, LIU Yun-guo, HU Xin-jiang. Study on the impact of chromium (VI) waste water migrating along river waterfront—A case study of the Chang-Zhu-Tan section of the Xiangjiang river [J]. Journal of Hunan University: Natural Sciences, 2012, 39(9): 80-86. (in Chinese)
[11] YAIMARA S, NATALIA D, RAUL G C, JENY C, ANDY H, MIRIELA T, RUTH E C, CALOS P. Preparation, characterization, and in vitro evaluation of nanostructured chitosan/apatite and chitosan/Si-doped apatite composites [J]. Journal of Materials Science, 2013, 48: 841-849.
[12] HU X J, WANG J S, LIU Y G, LI X, ZENG G M, BAO Z L, ZENG X X, CHEN A W, LONG F. Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics [J]. Journal of Hazardous Material, 2011, 185: 306-314.
[13] HONG Y, ZHONG Z H. Kinetics of adsorption of Zn2+ on imprinted chitosan polymer [J]. Chemical Industry and Engineering Progress, 2011, 30: 1290-1301.
[14] YU Zhou. Adsorption characteristics of heavy metal in groundwater adsorbed by modified chitosan [D]. Shanghai: Shanghai Jiao Tong University, 2010. (in Chinese)
[15] LI Zeng-xin, MENG Yun, WANG Tong, ZHANG Dao-lai. Enhanced extraction of chitosan for chromium(VI) from cantaminated soil [J]. Chinese Journal of Soil Science, 2009, 40(3): 660-663. (in Chinese)
[16] LIN Yun-hui, LIN Wen-fan, JIANG Kai-ning, LIN Pei-yu, LEI Meng-chuan. Adsorption with biodegradation for decolorization of reactive black 5 by Funalia trogii 200800 on a fly ash-chitosan medium in a fluidized bed bioreactor-kinetic model and reactor performance [J]. Biodegradation, 2013, 24(13): 7-15.
[17] LIU Ya-ting, LIU Yun-hai, CAO Xiao-hong, HUA Rong, WANG You-qun, PANG Cui, HUA Ming, LI Xiao. Biosorption studies of uranium (VI) on cross-linked chitosan: Isotherm, kinetic and thermo kinetic aspects [J]. Journal of Radioanalytical and Nuclear Chemistry, 2011, 290(2): 231-239.
[18] NAGAN P, SRINIVASAN L, PERSU N S, RENGANATHAN N G. Influence of clay on the adsorption of heavy metals like copper and cadmium on chitosan [J]. Environmental Science and Pollution Research, 2013, 20: 925-938.
[19] HO Y S, MCKAY G. Sorption of dye from aqueous solution by peat [J]. Chemical Engineering Journal, 1998, 70: 115-124.
[20] HO Y S. Review of second-order models for adsorption systems [J]. Journal of Hazardous Materials, 2006, 136: 681-689.
刘韵琴1,2,刘云国1, 胡新将1, 郭一明1
1. 湖南大学 环境科学与工程学院,长沙 410082;
2. 湖南商务职业技术学院 人文旅游系,长沙 410205
摘 要:利用分子印迹技术和甲基丙烯酸对壳聚糖进行改性,并在改变吸附条件、吸附动力学和吸附等温线的基础上,对湘江样水中Cr(VI)进行吸附研究。结果表明:X射线衍射谱显示印迹聚合物的结晶能力减弱,但非结晶区面积增加,吸附点位数提高,对Cr(VI)的吸附容量增大;印迹聚合物对Cr(VI)的吸附能力随时间的延长而增加,8 h后达到饱和,最佳吸附时间是吸附后4~8 h,对Cr(VI)的提取率最大值为33.7%。提取液最佳pH 值是4.5~7.5;提取率随着壳聚糖脱乙酰度的增大而增大,吸附效果最好的是90%脱乙酰度壳聚糖。吸附量随着壳聚糖的浓度增加而增加,饱和后对Cr(VI)的提取率变化相对平稳,实验测得最高去除率为98.3%。Cr(VI)印迹壳聚糖吸附的准一级动力学和二级动力学模型线性相关系数分别是0.9013和0.9875,吸附速率分别为0.0091 min-1和7.129 g/(mg·min)。Cr(VI)印迹壳聚糖的吸附更符合二级动力学模型,与Langmuir 吸附等温线的拟合性比 Freundlich吸附等温线的更好,计算得到的最大吸附容量为 15.784 mg/g,对河水中Cr(VI)的吸附效果明显。
关键词:改性壳聚糖;印迹;重金属污染物;Cr(VI);吸附;去除率;动力学模型
(Edited by Hua YANG)
Foundation item: Project (41271332) supported by the National Natural Science Foundation of China; Project (2010YBB186) supported by the Social Science Foundation of Hunan Province, Chian
Corresponding author: Yun-guo LIU; Tel: +86-731-88649208; E-mail: lyq521125@126.com; liuyunguo@hnu.cn
DOI: 10.1016/S1003-6326(13)62839-3

