Article ID: 1003-6326(2005)03-0583-06
Optimized synthesis technology of LiFePO4 for Li-ion battery
QU Tao(曲 涛), TIAN Yan-wen(田彦文), DING Yang(丁 扬),
ZHONG Can-yun(钟参云), ZHAI Yu-chun(翟玉春)
(School of Materials and Metallurgy, North-Eastern University,
Shenyang 110004, China)
Abstract: The influence of factors of the carbon black content, sintering temperature, sintering time, molar ratio of Li to Fe, as well as the electrochemical properties of LiFePO4 for lithium ion battery were studied. The only technology was obtained by using range analysis through Latin orthogonal experiment of L44(16). The results show that the optimization synthesis technology of LiFePO4 is content of 5% doping carbon, sintering temperature of 700℃, molar ratio of Li to Fe of 1.03∶1 and sintering time of 16h.The optimized cathode synthesis techniques can make LiFePO4 have good electrochemical properties.
Key words: lithium ion battery; cathode material; LiFePO4; orthogonal test CLC
number: TM912.9 Document code: A
1 INTRODUCTION
The interest in lithium rechargeable batteries in electric vehicles has been significantly increased in recent years[1, 2]. The important factors for their application are low price, long cycle life, environmental safety and high specific capacity.
Among typical Li secondary battery cathode materials, such as LiCoO2, LiMn2O4 and LiFePO4, LiFePO4 proposed by Padhi et al[3, 4] has attracted particular attention due to its low cost, environmental benignity, cycling stability and high temperature capability. Its main problems are low electronic conductivity and low lithium diffusivity[5]. Many research groups have tried to improve the performance of LiFePO4.The conductivity problems have been studied by optimization of particles . Yamada et al[6] identified the undesired particle growth over 600℃ and the presence of a residual Fe3+ phase below 500℃ as two extrinsic obstacles for achieving optimum charge/discharge performance. Other methods include metal doping[7, 8](adding Ag or Cu), coating with the electronically conductive materials like carbon[9-12], metal oxide[13, 14] and low temperature liquid phase synthesis[15].
Of all the methods, synthesis of LiFePO4 doping carbon black by solid-state reaction is a simple and effective way. Some research group only explored isolated factors, such as content of carbon black[11] or sintering temperature[16] that contributed to the electrochemical properties of LiFePO4. And the combined effect of sintering temperature, sintering time, content of carbon black and ratio of Li to Fe in solid-state synthesis LiFePO4 has not been reported yet.
In this paper, the influence of these four factors was studied, the electrochemical properties of LiFePO4 for lithium ion battery in solid-state reaction were tested and the optimized technology was found.
2 EXPERIMENTAL
2.1 Synthesis
LiFePO4 was prepared by solid-state reaction of FeC2O4·2H2O(AR), (NH4)2HPO4(AR) and Li2CO3(AR). A stoichiometric mixture of the raw materials and the measured amount of carbon black was dispersed into acetone and then thoroughly mixed and reground. After evaporating the acetone, the olivine phase LiFePO4 was synthesized in a purified Ar gas flow to prevent the formation of Fe3+ compounds as impurities; the mixture was first decomposed at 350℃ for 8h to expel the gases and reground, then sintered under a given temperature schedule.
2.2 Characterization
The X-ray diffraction(XRD) with CuKα radiation was used to identify the phases. Scanning electron microscope(SEM) images were observed using the JSM-5800.
2.3 Electrochemical test
The performance of LiFePO4 as cathode was evaluated using an experimental cell with a lithium metal anode. The cathode was a mixture of LiFePO4/acetylene black /polyvinylidene fluoride(PVDF) with mass ratio 70∶20∶10; a micro-porous polypropylene film(Celgard 2400) was used as a separator and the electrolyte was a 1mol/L LiPF6-propylene carbonate/dimethy carbonate(PC/DMC) solution. All cells were assembled inside a glove box filled with purified argon. The galvanostatic charge-discharge experiment was performed between 2.6V and 4.1V, at the rate of C/10, using a Dc-5computer-controlled galvanostat and potentiostat.
2.4 Single factor experiment
The first heating step was to decompose the mixture and expel the gases, so we focused on the second heating step .The four single factor experiments are as follows:
1) Sintering time(24h), sintering temperature(700℃) and molar ratio of Li to Fe(1.05∶1) were settled. And content of doping carbon black was fixed at 0%, 1%, 3%, 5%, 7%.
2) The content of doping carbon(5%), sintering time(24h) and molar ratio of Li to Fe(1.05∶1) were settled. And sintering temperature was fixed at 600, 650, 700, 750 and 800℃.
3) The content of doping carbon(5%), sintering temperature(700℃), and molar ratio of Li to Fe(1.05∶1) were settled. And sintering time was fixed at 16, 20, 24, 28 and 32h.
4) The content of doping carbon(5%), sintering time(24h), and sintering temperature(700℃) were settled. And the molar ratio of Li to Fe was fixed at 1∶1, 1.03∶1, 1.05∶1, 1.08∶1 and 1.1∶1.
2.5 Orthogonal test
In order to obtain optimum technological conditions of synthesizing LiFePO4, L44(16) Latin orthogonal test was performed[17] based on single factor experiments .The target was the first discharge capacity. The factors and levels of orthogonal test are shown in Table 1.
3 RESULTS AND DISCUSSIONS
3.1 Single factor experiment
The results of single factor experiments are listed in Table 2.
3.1.1 Effect of content of carbon black on initial rate capacity
The conductivity of LiFePO4 is poor. The addition of carbon during the synthesis of LiFePO4 increases the conductivity, improves the electrochemical performance of the material in terms of practical discharge capacity[18, 19], and reduces the grain size of the active material. The reduction in the grain size can be related to the fact that the carbon particles, uniformly distributed between the starting materials, can interfere with the grains coalescence. The smaller the grain size, the higher current densities it can support. The electronic contact between the grains is enhanced because the conductive filler interacts with the grains during their formation. When the sintering temperature,
Table 1 Factors and levels for orthogonal experiment
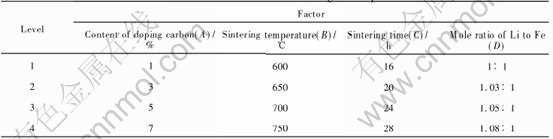
Table 2 Results of single factor experiment
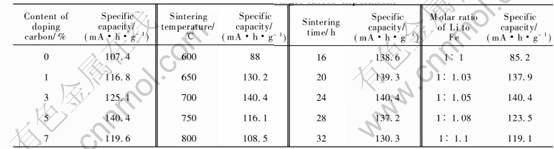
sintering time and the molar ratio of Li to Fe were fixed, the initial discharge capacity stepped up with the increment of carbon black. But when the content of carbon is more than 5%, the reduction of grain size is not notable, the proportion of active material decreases; so the initial rate capacity of LiFePO4 cannot be improved.
3.1.2 Effect of sintering temperature on initial rate capacity
When the content of carbon, sintering time and the molar ratio of Li to Fe were constant, the initial discharge capacity of LiFePO4 reached its peak as samples were prepared at 700℃. That is because the crystals grow maturely as the temperature gets up until 700℃. When the sintering temperature is over 700℃, the particles grow excessively large. This causes less specific area of particles, fewer tunnels for Li ions motion, and less discharge capacity.
3.1.3 Effect of sintering time on initial rate capacity
When the sintering temperature, content of carbon black and molar ratio of Li to Fe were fixed, the initial rate capacity of LiFePO4 didnt seem to change a lot until the sintering time got to 28h. This illustrates that LiFePO4 grows excessively large after too-long sintering time.
3.1.4 Effect of molar ratio of Li to Fe on initial rate capacity
When the content of carbon black, sintering time and sintering temperature were fixed, the initial rate capacity went up along with the increase of molar ratio of Li to Fe until it reached 1.05∶1.The main reason is that Li2O decomposed from Li2CO3 is inclined to volatilize, which causes the loss of lithium. But excessive molar ratio of Li to Fe could not meet the stoichiometric proportion of LiFePO4 either. The only ratio of Li to Fe is 1.05∶1.
3.2 Orthogonal test
Table 2 shows the factors and levels of the orthogonal test for synthesizing the cathode material LiFePO4. And Table 3 shows the result and range
Table 3 Result and range analysis of orthogonal test
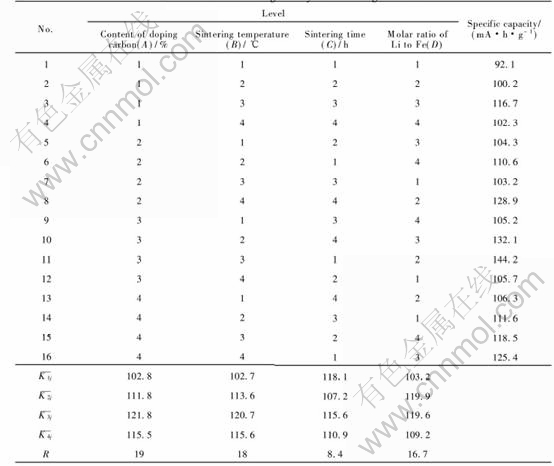
analysis of the orthogonal test. The figure of R(range) represents the significance of each factor in the experiments. The larger the R(range), the more the factor contributes to the capacity. And the smaller one means that the factor has less weight on the index. According to R(range), the sequence of the weight of these four factors on the initial specific capability is A→B→D→C. The content of carbon and sintering temperature are two most important factors .The molar ratio of Li to Fe follows. And sintering time has the least significance. From Fig.1, as to factor A, if the content of carbon exceeds level 3, the initial discharge capability will not increase according to the single factor experiment. So A3 is the best choice. As to factor B, if sintering temperature surpasses level 3, the rate capability will not increase according to the single factor experiment as well. So B3 is chosen. As to factor C, since it has a minimum significance, the influence of sintering time on synthesizing LiFePO4 is least. A sintering time that can satisfy a good crystal development is enough. Considering the result of single factor experiment, C1 is adopted. As to factor D, the figures of levels 2 and 3 are very similar, which illustrates that there seems to be a platform between 1.03∶1 and 1.05∶1. Considering the cost of the production, D2 is selected. Judging from the orthogonal test, the optimized technology for synthesizing the cathode material LiFePO4 is A3B3C1D2.
3.3 Confirmation test
Olivine LiFeO4 was synthesized by solid-state reaction. The molar ratio for FeC2O4·2H2O(AR)∶(NH4)2HPO4(AR)∶Li2CO3(AR) was 1∶1∶0.515. The raw materials and carbon black(5%) were dispersed into acetone and then thoroughly mixed and reground. After evaporating the acetone, the mixture was first decomposed at 350℃ for 8h to expel the gases and reground, then sintered at 700℃ for 16h. All the sintering processes were in Ar gas flow.
The crystal phase of the sample was identified to be LiFePO4 phase with ordered olivine structure indexed by orthorhombic Pnmb(as shown in Fig.2). Fig.3 shows SEM image of the sample
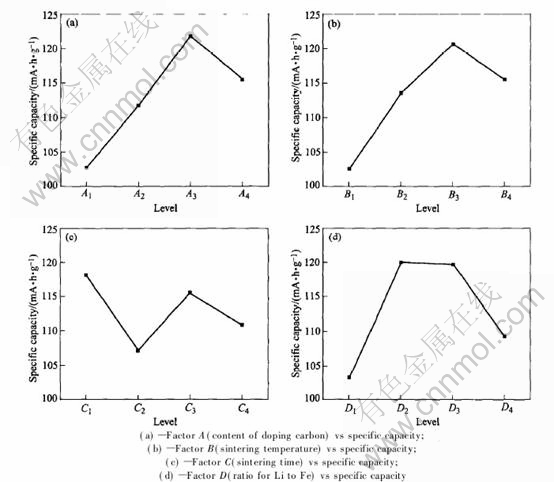
Fig.1 Relationships between factors and target in orthogonal test
of LiFePO4 prepared in optimized technology. To test its electrochemical performance, experimental batteries were assembled by using the method of section 2.3. The result of electrochemical test shows that initial discharge capacity reaches 143.5mA·h/g(Fig.4), which matches the result of the orthogonal test.
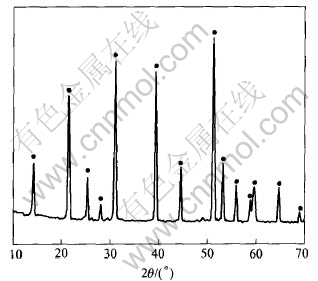
Fig.2 XRD pattern of LiFePO4 prepared in optimized technology
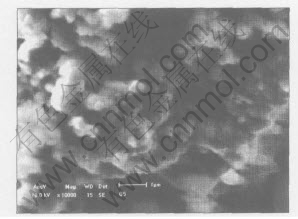
Fig.3 Morphology of LiFePO4 prepared in optimized technology
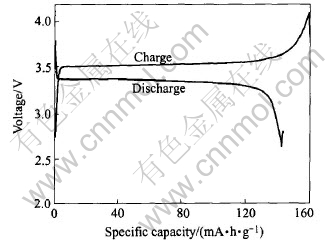
Fig.4 Charge/discharge curves of LiFePO4 prepared in optimized technology at room temperature
4 CONCLUSIONS
1) The sequence of factors that can influence the initial rate capacity of LiFePO4 is: content of doping carbon black, sintering temperature, molar ratio for Li to Fe, sintering time. The mass of carbon black and sintering temperature are two most important factors. The molar ratio of Li to Fe follows. And sintering time has the minimum significance. Certain of the only synthesis technology of LiFePO4 is content of 5% doping carbon, sintering temperature of 700℃, mole ratio of Li to Fe of 1.03∶1 and sintering time of 16h.
2) The confirmation tests show that initial discharge capacity reaches 143.5mAh/g, which matches the result of the orthogonal test. The specific capacity of the result is higher than that of the industrialized LiCoO2. LiFePO4 got from optimized synthesis technology has a promising applied future.
REFERENCES
[1]Duong T Q. USABC and PNGV test procedures [J]. J Power Sources, 2000, 89(2): 244-248.
[2]Zhang X, Ross P N, Kostecki R, et al . Diagnostic characterization of high power lithium-ion butteries for use in hybrid electric vehicles [J]. J Electrochem Soc, 2001, 148(5): A463-A470.
[3]Padhi A K, Nanjundawamy K S, Goodenough J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries [J]. J Electrochem Soc, 1997, 144(4): 1188-1194.
[4]Padhi A K, Nanjundawamy K S, Goodenough J B, et al. Effect of structure on the Fe3+/Fe2+ redox couple in iron phosphates [J]. J Electrochem Soc, 1997, 144(5): 1609-1613.
[5]Andersson A S, Thomas J O. The source of first-cycle capacity loss in LiFePO4 [J]. J Power Sources, 2001, 97-98: 498-502.
[6]Yamada A, Chung S C, Hinokuma K. Optimized LiFePO4 for lithium battery cathodes [J]. J Electrchem Soc, 2001, 148(3): A224-A229.
[7]Croce F, Epifanio A D, Hassoun J, et al . A novel concept for the synthesis of an improved LiFePO4 lithium battery cathode [J]. Electrochem and Solid State Lett , 2002, 5(3): A47-A50.
[8]Park K S, Son J T, Chung H T, et al. Surface modification by silver coating for improving electrochemical properties of LiFePO4 [J].Solid State Communications, 2004, 129(5): 311-314.
[9]Prosini P P, Zane D, Pasquali M. Improved electrochemical performance of a LiFePO4 based composite cathode [J ]. Electrochimica Acta, 2001, 46(23): 3517-3523.
[10]Huang H, Yin S C, Nazar L F. Approaching theoretical capacity of LiFePO4 at room temperature at high rates [J] . Electrochem and Solid State Lett , 2001, 4(10): A170 - A172.
[11]Chen Z, Dahn J R. Reducing carbon in LiFePO4/ C composite electrodes to maximize specific energy , volumetric energy , and tap density [J ]. J Electrochem Soc, 2002, 149(9): A1184 - A1189.
[12]Park K S, Son J T, Chung H T, et al. Synthesis of LiFePO4 by co-precipitation and microwave heating [J]. Electrochemistry Communications, 2003, 5(10): 839-842.
[13]Chung S Y, Blocking J T, Chiang Y M. Electronically conductive phospho-olivines as lithium storage electrodes [J] . Nature Mater, 2002, 2: 123 - 128.
[14]Barker J, Saidi M Y, Swoyer J L. Lithium iron(Ⅱ): phospho-olivines prepared by a novel carbothermal reaction method [J]. Electrochem and Solid State Lett, 2003, 6(3): A53-A55.
[15]Iltchev N, Chen Y, Okada S, et al. LiFePO4 storage at room and elevated temperatures [J]. J Power Sources, 2003, 119-121: 749-754.
[16]Kim H S, Cho B W, Cho W I. Cycling performance of LiFePO4 cathode material for lithium secondary batteries [J]. J Power Sources, 2004, 132(1-2): 235-239.
[17]Groups of compiling orthogonal test. Method of Orthogonal Test [M]. Beijing: National Defence Industry Press, 1976. 185-187.
[18]Bewlay S L, Konstantinov K, Wang G X, et al. Conductivity improvements to spray-produced LiFePO4 by addition of a carbon source [J]. Materials Letters, 2004, 58(11): 1788-1791.
[19]Chung S Y, Chiang Y M. Microscale measurements of the electrical conductivity of doped LiFePO4 [J]. Electrochem and Solid State Lett, 2003, 6(12): A278 - A281.
(Edited by LI Xiang-qun)
Foundation item: Project(9810300702) supported by the Natural Science Foundation of Liaoning Province, China
Received date: 2004-07-02; Accepted date: 2004-12-06
Correspondence: TIAN Yan-wen, Professor; Tel: +86-24-83687731; E-mail: qvtao@126.com