
Fabrication and photocatalytical properties of flower-like TiO2 nanostructures
LIU Min(刘 敏), LU Wei-ming(鲁伟明), ZHAO Lei(赵 雷), ZHOU Chun-lan(周春兰),
LI Hai-ling(李海玲), WANG Wen-jing(王文静)
Key Laboratory of Solar Thermal Energy and Photovoltaic System, Institute of Electrical Engineering,
Chinese Academy of Sciences, Beijing 100190, China
Received 13 July 2009; accepted 4 February 2010
Abstract: Three-dimensional (3D) flower-like anatase TiO2 nanostructures and flower-like titanate nanostructures were successfully synthesized via hydrothermal synthesis followed by post-treatment from titanium powder. The flower-like anatase TiO2 nanostructures were characterized in detail with scanning electron microscopy (SEM), X-ray diffraction (XRD), UV-vis spectrum and nitrogen adsorption-desorption measurement, respectively. It is found that the flower-like TiO2 nanostructures have a high specific surface area and a large light-harvesting efficiency. The photocatalytical activity of the flower-like anatase TiO2 nanostructures was determined by degradation of methylene blue in aqueous solution, and was compared with commercial P25 titania. It is revealed that the photocatalytical activity of the flower-like anatase TiO2 nanostructures is enhanced a lot. The apparent rate constant of the flower-like anatase TiO2 nanostructures is almost 2 times that of P25 titania.
Key words: Ti powder; hydrothermal synthesis; flower-like; TiO2; photocatalysis
1 Introduction
Titanium dioxide (TiO2) is one of the most important transition metal oxides which has been widely used in catalysis, photovoltaic cells, self-cleaning devices, sensors, Li-ion battery materials, optical emission, water-splitting, paints, paper, cosmetics, and so on[1-4]. It is found that the application of TiO2-based devices is strongly dependent on its own crystalline structure, morphology and phase dimension[3-7]. Therefore, the controlled synthesis of TiO2 nanostructures with different shapes and sizes has been developed in recent years. So far, most synthetic efforts have been directed toward monodisperse nanoparticles, nanotubes, nanowires, and nanoribbons. The synthesis of 0-dimensional (0D) and one-dimensional (1D) nanostructures has been widely investigated and well developed. However, reports on the synthesis of complex three-dimensional (3D) titania nanostructures remain uncommon. On the other hand, recent research on the synthesis of 3D nanomaterials shows that this kind of nanostructure may have potentially to explore their novel properties[8-11]. For example, flower-like TiO2 nanostructures, which were synthesized by a hydrothermal method using TiCl4 as raw material, exhibited enhanced light adsorption and photocatalysis[10]. Brookite TiO2 nano?owers, which were synthesized by a hydrothermal method from TiOSO4, had larger permittivity than anatase TiO2 products[11]. However, flower-like TiO2 nanostructures, which are fabricated from Ti powders through a hydrothermal method, to the best of our knowledge, have never been reported.
In this work, we report a facile hydrothermal synthesis of flower-like anatase TiO2 nanostructures and flower-like titanate nanostructures, which are composed of nanoribbons, from titanium powder. The prepared flower-like anatase TiO2 nanostructures have a high specific surface and a large light-harvesting efficiency and. And they exhibited high photocatalytical activity for degradation of methylene blue (MB).
2 Experimental
Flower-like anatase TiO2 nanostructures were fabricated through a hydrothermal reaction between NaOH solution and the mixture of Ti powders, H2O2 and HNO3, followed by heat treatment. In a typical procedure, 0.1 g Ti powders (~80 μm, 99.9% purity, Beijing Chemical Factory) were dissolved in 150 mL 9 mol/L H2O2+0.1 mol/L HNO3 aqueous solution at 80 °C for 2 h. After the solution was cooled to the room temperature naturally, 5 mL such solution was mixed with 15 mL concentrated NaOH solution (40%, mass fraction, Tianjin Fengchuang Chemical Factory, China). Then, the mixed solution was placed in a Teflon-lined autoclave and was kept at 150 °C for 3 h. After that, the products were collected by centrifugation and thoroughly washed with high purity water (18 MΩ) and 0.1 mol/L HNO3 aqueous solution, until pH was 7.0. Finally, the white product was annealed at 450 °C for 2 h in air.
The phase identification of the samples was conducted with powder X-ray diffractometer (XRD, Bruker D8 Advance, Cu Kα radiation λ=1.540 56 ?). The morphologies and composition of the samples were observed on field-emission scanning electron microscope (FE-SEM, Hitachi S-4800 with energy disperse spectroscope (EDS) capabilities). The UV–vis absorption spectra of the samples were observed with Varian Cary 5 000 equipped with an integrating sphere. The Brunauer–Emmett–Teller (BET) surface area measurements were carried out by N2 adsorption at 77 K using an ASAP2020 instrument.
Photocatalytical activity of the flower-like anatase TiO2 nanostructures was evaluated in terms of the decolorization MB dye under ultraviolet (UV) irradiation. flower-like anatase TiO2 sample of 10 mg was dispersed into 100 mL 10 mg/L MB solution and stirred in the dark for 2 h to reach a complete adsorption-desorption equilibrium. Then the solution was irradiated with ~0.5 mW/cm2 UV light (with a wavelength peak at 365 nm) under continuous stirring. With a given irradiation time interval, some specimens (5 mL) were taken from the dispersion and were centrifuged (4 000 r/min). The clear upper solution was subjected to an UV-Vis spectrophotometer (Shanghai Spectrum Instruments Co., Ltd., WFJ721E). The concentration of MB was determined from the absorbance at the wavelength of 665 nm. For comparison, P-25 was used as the benchmark to evaluate the photocatalytical activity of the flower-like TiO2 nanostructures. P-25 is a mixture of anatase (~79%) and rutile (~21%) TiO2 and it is currently considered to be one of the best commercial TiO2 photocatalysts.
3 Results and discussion
Typical FE-SEM images of flower-like titanate nanostructures and flower-like TiO2 nanostructures are shown in Fig.1. As shown in Fig.1(a), the titanate product contains numerous flower-like nanostructures. These flowers are composed of nanoribbons. Fig.1(b) shows the SEM image of the flower-like TiO2 nanostructures. It reveals that moderate high-temperature annealing dehydration process does not destroy the 3D hierarchical structural motif of the flower-like TiO2 nanostructures. The TiO2 nanostructures maintain the flower-like morphology. Energy dispersive spectrometer (EDS) analysis (inset of Fig.1(b)) shows that flower-like titania nanostructures are chemically composed of Ti, O elements (C element was originated from conductive carbon tapes and Si element was originated from Si substrate). This demonstrates that complete ionic exchange between Na+ and H+ is achieved.

Fig.1 FE-SEM images of flower-like titanate nanostructures (a) and anatase flower-like TiO2 nanostructures (b) (inset: EDS spectrum of flower-like TiO2 nanostructures)
The corresponding XRD patterns recorded from flower-like titanate nanostructures and flower-like TiO2 nanostructures are shown in Fig.2. The crystal of the products before annealing treatment is not very fine. According to the previous studies, the observed 2θ values for the XRD at 9.1°, 24.5°, 48.3°, and 62.5° can be attributed to (020), (110), (200), and (002) faces of H2Ti3O7, respectively[12-13]. This XRD result suggests that the flower-like nanostructures is a kind of layered titanate. After being annealed at 450 °C in air for 2 h, all the relatively sharp peaks could be indexed as anatase TiO2, which are in good agreement with the reported values of Joint Committee on Powder Diffraction Standards (JCPDS) card No. 21-1272. There are no characteristic peaks of impurities, such as sodium titanium oxide, conforming that Na+ is removed completely.
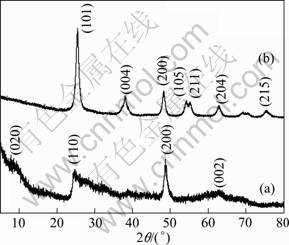
Fig.2 XRD patterns of flower-like titanate nanostructures (a) and flower-like anatase TiO2 nanostructures (b)
The surface area and the porosity of the flower-like anatase TiO2 nanostructures were measured by nitrogen adsorption-desorption. According to the Brunauer– Emmett–Teller (BET) method, the specific surface area of the flower-like TiO2 nanostructures is 134.7 m?/g, which is higher than that of Degussa P25, ~50 m?/g. And the pore volume of the flower-like TiO2 nanostructures is 0.21 cm3/g. Fig.3 shows the pore size distribution plot, which was obtained by using the Barrett-Joyner-Halenda (BJH) method (dV/dD is pore volume of sample). It can be seen that the flower-like TiO2 nanostructure has a narrow pore-size distribution with an average pore diameter of ca. 42.1 nm. This high porous structures with high specific surface area is beneficial to light-harvesting and mass transport[14-19].
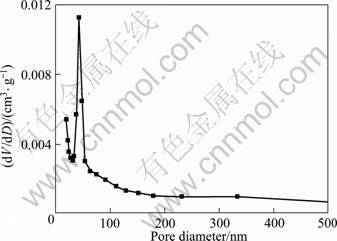
Fig.3 Pore size distribution of flower-like anatase TiO2 nanostructures
The UV-visible absorption spectra of the flower-like anatase TiO2 nanostructures and commercially-available P25 titania are shown in Fig.4. It can be seen from Fig.4 that the absorption of the flower-like TiO2 nanostructures is stronger than that of P25. This may be due to the porous structure of the flower-like TiO2 nanostructures, which is believed to favor the harvesting of light due to multiple scattering of light within the porous framework[14-16]. Therefore, the light-harvesting efficiency of flower-like TiO2 nanostructures would be larger than that of P25.

Fig.4 UV-visible absorption spectra of P25 and flower-like anatase TiO2 nanostructures
The photocatalytical activity of the flower-like TiO2 nanostructures was further studied. Fig.5(a) presents the variation of MB concentration by recording the UV-vis spectra at interval of 10 min during the photochemical degradation of MB on the flower-like anatase TiO2 nanostructures and P-25 TiO2 under UV irradiation. The linear relationship of ln ρ0/ρ vs time (Fig.5(b)) shows that the photocatalytical degradation of MB follows the pseudo-first-order kinetics:
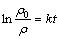 (1)
(1)
where ρ0/ρ is the normalized MB concentration; t is the reaction time; and k is the apparent reaction rate in terms of min-1. The apparent photochemical degradation rate constant for the flower-like anatase TiO2 nanostructures is 2.25×10-2 min-1, which is almost 2 times that for the P-25, 1.21×10-2 min-1, further confirming that the flower-like anatase TiO2 nanostructures exhibit high photocatalytical efficiency. The enhanced photocatalytical activity of the flower-like anatase TiO2 nanostructures can be attributed to their high surface area and large light-harvesting efficiency. Usually, a large surface area can offer more active adsorption sites and photocatalytical reaction centers[17-19]. Moreover, the porous structure is believed to favor the light-harvesting and mass transport[14-16]. All of these enhance the rate of photocatalytical reaction.
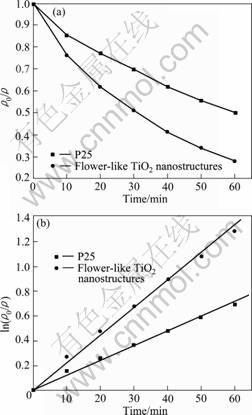
Fig.5 Variation of MB concentration by photochemical reaction with P-25 TiO2 and flower-like anatase TiO2 nanostructures under UV irradiation (a) and pseudo-first-order kinetic rate for photochemical degradation of MB (b)
4 Conclusions
1) A simple, inexpensive, and mild synthetic process is reported to synthesize flower-like anatase TiO2 nanostructures from Ti powder.
2) The synthesized flower-like TiO2 nanostructures have a high BET surface area of 134.7 m2/g.
3) The flower-like TiO2 nanostructures exhibit a large light-harvesting efficiency.
4) The photocatalytical efficiency of the flower-like TiO2 nanostructures is almost 2 times that of P25 titania.
5) Owing to their large specific surface area and high photocatalytical activity, the flower-like TiO2 nanostructures can be further applied in the areas of separation technology, solar cells, sensors, Li-ion electrode materials and so on.
References
[1] FUJISHIMA A, HONDA K. Electrochemical photolysis of water at a semiconductor electrode [J]. Nature, 1972, 238: 37-38.
[2] O'REGAN B, GRATZEL M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films [J]. Nature, 1991, 353: 737-740.
[3] LINSEBIGLER A L, LU Gap-qing, YATES J T. Photocatalysis on TiO2 surface: Principles, mechanisms and selected results [J].Chem Rev, 1995, 95(3): 735-758.
[4] KUCHIBHATLA S V N T, KARAKOTI A S, BERA D, SEAL C. One dimensional nanostructured materials [J]. Prog Mater Sci, 2007, 52: 699-913.
[5] HUANG Ji-quan, HUANG Zhi, GUO Wang, WANG Mei-li, CAO Yong-ge, HONG Mao-chun. Facile synthesis of titanate nanoflowers by a hydrothermal route [J]. Crystal Growth & Design, 2008, 8(7): 2444-2446.
[6] TOKUDOME H, MIYAUCHI M. Electrochromism of titanate-based nanotubes [J]. Angew Chem Int Ed, 2005, 44(13):1974-1977.
[7] WANG Bao-xiang, SHI Yong, XUE Dong-feng. Large aspect ratio titanate nanowire prepared by monodispersed titania submicron sphere via simple wet-chemical reactions [J]. J Solid State Chem 2007, 180: 1028-1037.
[8] LUO Yong-song, LI Su-qin, REN Qin-feng, LIU Jin-ping, XING Lan-lan, WANG Yan, YU Ying, JIA Zhi-jie, LI Jia-lin. Facile synthesis of flowerlike Cu2O nanoarchitectures by a solution phase route [J]. Cryst Growth Des, 2007, 7(1): 87-92.
[9] FANG Xiao-sheng, YE Chang-Hui, ZHANG Li-de, ZHANG Jun-xi, ZHAO Jian-wei, YAN Peng. Direct observation of the growth process of MgO nanoflowers by a simple chemical route [J]. Small, 2005, 1(4): 422-428.
[10] ZHU Jian, WANG Shao-hua, BIAN Zhen-feng, CAI Chen-ling, LI He-xing. A facile synthesis of hierarchical ?ower-like TiO2 with enhanced photocatalytic activity [J]. Res Chem Intermed, 2009, 35: 769-777.
[11] HU Wan-biao, LI Li-ping, LI Guang-she, TANG Chang-lin, SUN Lang. High-quality brookite TiO2 flowers: Synthesis, characterization, and dielectric performance [J]. Cryst Growth Des, 2009, 9(8): 3676-3682.
[12] WU Zhong-biao, DONG Fan ZHAO Wei-rong, WANG Hai-qiang, LIU Yue, GUAN Bao-hong.ited by LI Xiang-qun The fabrication and characterization of novel carbon doped TiO2 nanotubes, nanowires and nanorods with high visible light photocatalytic activity [J]. Nanotechnology, 2009, 20: 235701-1-9.
[13] MAO Yuan-bing, WONG S S. Size- and shape-dependent transformation of nanosized titanate into analogous anatase titania nanostructures [J]. J Am Chem Soc, 2006, 128(25): 8217-8226.
[14] LI F B, LI X Z, HOU M F, CHEAH K W, CHOY W C H. Enhanced photocatalytic activity of Ce3+-TiO2 for 2-mercaptobenzothiazole degradation in aqueous suspension for odour control [J]. Appl Catal A, 2005, 285: 181-189.
[15] YU Jia-guo, WANG Guo-hong, CHENG Bei, ZHOU Ming-hua. Effects of hydrothermal temperature and time on the photocatalytic activity and microstructures of bimodal mesoporous TiO2 powders [J]. Appl Catal B, 2007, 69: 171-180.
[16] WANG Xin-chen, YU J C, CHEN Yi-lin, WU Ling, FU Xian-zhi. ZrO2-modified mesoporous nanocrystalline TiO2-xNx as efficient visible light photocatalysts [J]. Environ Sci Technol, 2006, 40(7): 2369-2374.
[17] AO Yan-hui, XU Jing-jing, FU De-gang, YUAN Chun-wei. A simple method for the preparation of titania hollow sphere [J]. Catal Commun, 2008, 9(15): 2574-2577.
[18] YU Jia-guo, LIU Sheng-wei, YU Huo-gen. Microstructures and photoactivity of mesoporous anatase hollow microspheres fabricated by fluoride-mediated self-transformation[J]. J Catal, 2007, 249: 59-66.
[19] YU Jia-guo, YU J C, LEUNG M K P, HO W K, CHENG Bei, ZHOU Xiu-jian, ZHAO Jin-cai. Effects of acidic and basic hydrolysis catalysts on the photocatalytic activity and microstructures of bimodal mesoporous titania [J]. J Catal, 2003, 217(1): 69-78.
(Edited by LI Xiang-qun)
Foundation item: Project(60576065) supported by the National Natural Science Foundation of China; Projects(2006AA05Z405, 2006AA04Z345) supported by the National High-tech Research and Development Programme of China; Project(KGCXZ-YW-351) supported by Knowledge Innovation Program of the Chinese Academy of Sciences, China
Corresponding author: WANG Wen-jing; Tel: +86-10-82547042; E-mail: wjwangwj@126.com
DOI: 10.1016/S1003-6326(10)60644-9