J. Cent. South Univ. Technol. (2008) 15: 469-473
DOI: 10.1007/s11771-008-0088-6
Synthesis of optically active lactide catalyzed by nanocrystals of La-Ti composite oxides
GU Li(谷 俐), LI Fei(李 菲), MA Hong-xia(马红霞), ZHANG Yang(张 洋)
(School of Biology and Chemical Engineering, Jiaxing University, Jiaxing 314001, China)
Abstract: With sol-gel method, nanometer La-Ti composite oxides were prepared. By means of atomic force microscope, the surface pattern, particle size distribution and specific surface area were studied. The newly prepared nanocrystals of La-Ti composite oxides were used as the catalysts to catalyze the dehydration of external compensated lactic acid to lactide. The lactide product was measured by polarimeter and micropolariscope. The results demonstrate that the ratio between D-lactide and L-lactide will not be equal to 1?1 if nanocrystals of La-Ti composite oxides are used as the catalysts, which implies, that nanocrystals of La-Ti composite oxides may be potential catalysts with a good selectivity.
Key words: La-Ti composite oxides; lactic acid; lactide; catalysis; optical activity
1 Introduction
Due to good bioconsistence, biodegradability, and without poisoning side effect, polylactide (PLA) has attracted much attention recently. It shows wide applications in the field of drug controlling release system, tissue engineering, environmental materials, and so on[1-5]. Currently, PLA is synthesized mainly by the direct condensation polymerization of lactic acid or the ring-opening polymerization of lactide[6-8].
In order to get PLA with a high relative molecular mass, the method of the ring-opening polymerization of lactide is usually required. Recently, much research has been devoted to the increase of the productivity of lactide under the catalysis of various oxides by means of improving the synthesis of lactide[9-10], choosing better solvent for lactide purification[11-12], and trying different catalysts[13-18].
In this work, nanocrystals of La-Ti composite oxides were prepared via a sol-gel route, and then the newly prepared composite was used as catalysts to synthesize lactide. The crystal section features, optical rotation, and polarity of the lactide were studied. The phenomenon of micro-phase separation in the lactide was observed, which demonstrates that the nanocrystals of La-Ti composite oxides enjoy the ability of selective catalysis. The improvement of productivity of lactide will bring a bright future for the broad application of poly lactic acid.
2 Experimental
2.1 Materials
Lanthanum oxide, titanic butonate, polyethylene glycol(PEG) with a relative molecular mass of 5 000- 10 000, D-, L-lactic acid, acetic ester and zinc oxide were purchased from Shanghai Chemical Reagent Company.
2.2 Preparation and characterization of La-Ti composite oxides
The synthesis procedures were as follows: a weighed amount of La2O3 was first dissolved by nitric acid, and an appropriate amount of PEG was added into this La2O3 solution under stirring at ambient temperature, then a transparent solution sol (S1) formed. A stoichio- metric amount of (C4H9O)4Ti dispersed in ethanol by swift stirring, and a light yellow transparent solution sol (S2) formed. S2 was added dropwise in S1. After complete mixing, the sol was distilled to remove water entirely at 90 ℃ by vigorous stirring throughout the whole evaporation process. After dehydration, the residue formed a complete homogeneous transparent sol. The sol was slowly cooled to ambient temperature to form a milk white gel. The gel was desiccated in air at 100 ℃ for 10 h and the dry gel was obtained. Then the dry gel underwent subsequent heat treatment in air at 750 ℃ for 3 h, and bright white well-crystallized and ultrafine La-Ti oxides particles were obtained. The crystal structure and surface topography of the compound particle were investigated by (Rigaku-D/Max-RA) X-ray diffraction meter and CSPM-2000 atomic force microscope individually.
2.3 Preparation of lactides
In a typical process, 300 g D-, L-lactic acid was added to a 500 mL three-necked flask equipped with stirring system, vacuum-pumping system and cooling system. After removing most of free water at 60 ℃ and in vacuum, catalysts (zinc oxide or nanocrystals of La-Ti composite oxides) with a mass fraction of 1%-5% were added to the system. Then, the temperature of the system was increased to 120 ℃ to promote the formation of oligomer and the removal of the generating water. This process was continued until no water was distilled. After that, the temperature of the system was increased to 180-200 ℃ quickly. It can be found that large amount of yellow liquid was distilled and they crystallized into needle-like crystal after cooling. The products were re-crystallized for at least 4 times in order to get high-purity and colorless lactide. The reaction mechanism is as follows.
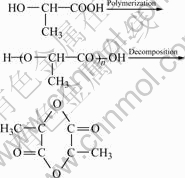 (1)
(1)
2.4 Characterizations of lactides
IR spectrum of the as-prepared lactide was recorded by a Nicolet-IR550 infrared spectrometer. The measurement of the optical rotation of the lactide was by a Rudolph-Autopol IV fully-automatic polarimeter (t= 26 ℃, λ=589 nm). The analysis of polarization of the lactide was performed by a Nikon eclipse-E400 micro- polariscope. SEM iamges of the lactide were taken by a Hitachi X-650 scanning electronic microscope(SEM).
3 Results and discussion
3.1 XRD pattern of La-Ti composite oxide
Fig.1 shows the XRD pattern of La-Ti composite oxides, the location and strength of the sample are in good concordance with the data of monoclinic crystals La2Ti2O7 and orthogonal crystals of La4Ti9O24 based on the JCPDS card, without any feature peak of other crystal.
3.2 Surface feature of La-Ti composite oxides
Fig.2 shows an ideal representation of the composite oxides surface. The surface sheet consists of well-shaped even particles, which appear in the form of round shape with clear-cut brim and accumulate closely to form an even flake (see Fig.2(a)). The three dimensional surface topography pattern shows the fluctuation of the compound surface (see Fig.2(b)), the surface is smooth and fluctuates a little and the largest height of the surface outline is 6.96 nm and the mean height of the outline is 4.69 nm, suggesting an accumulation of La-Ti composite oxide nanocrystals with a narrow and homogeneous particle size distribution.
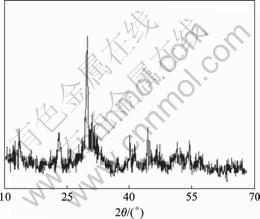
Fig.1 X-ray powder diffraction pattern of nanometer La-Ti composite oxides

Fig.2 AFM images of La-Ti composite oxide with scanning scope of 530.77 nm×530.77 nm: (a) Two-dimensional AFM image; (b) Three-dimensional AFM image
3.3 Catalytic activity of La-Ti composite oxides
Table 1 summarizes the experimental results in the preparation in the presence of ZnO or nanocrystals of La-Ti composite oxides and without any catalysts. In the experiments, if ZnO powder was selected as the catalysts, it could not be dispersed well in the reaction system and was inclined to aggregrate. This is not advantageous to the dehydration, and in particular, the viscosity of the system is rather large. So, in order to remove the water in the system, it is necessary to increase the temperature, which usually leads to the carbonization of parts of lactic acid. As a result, the producivity of lactide is decreased. However, contrary phenomena were observed if nano- crystals of La-Ti composite oxides were used as the catalysts. In the presence of La-Ti composite oxides, the time of dehydration of the system was shortened obviously and the viscosity of the system was decreased greatly. The producitivity of the lactides is about one and half times of that catalyzed by ZnO. It is becasue nanocrystals of La-Ti composite oxides can be dispersed homogenusously in the system and they did not precipitate from the solution. And every nanocrystasl can act as the boiling center, which increases the mobility of the system (namely decreases the viscosity of the system ) and diminishes side reactions.
Table 1 Catalytic activity of oxides in preparation of lactide

3.4 Chemical structure of lactide
Fig.3 shows the IR spectrum of the lactide. The peaks around 3 009.9 and 2 925.0 cm-1 originate from the dilation vibration of methine group and the symmetrical dilation vibrationand of methyl group, respectively. The peaks centered at 1 759.6 and 1 258.0 cm-1 are known as the dilation vibration of carbonyl and alkoxyl groups, respectively. In the fingerprint region more absorption bands were found, which is consistent with the structure of cycloester. Consequently, IR analysis confirms that the products are pure lactides.
3.5 Optical activity of lactide
Table 2 summarizes the optical activity of standard lactides and those prepared using ZnO and nanocrystals of La-Ti composite oxides as catalysts. It is interesting that the optical activity of the lactide catalyzed by nanocrystals of La-Ti composite oxides is about -20.015?, which demonstrates that the content of L-lactide is larger than that of D-lactide. The chirality of the as-prepared lactides is confirmed by the measuements of polarization. As shown in Fig.4, the phenomenon of polarized light of the crystals is rather obvious.
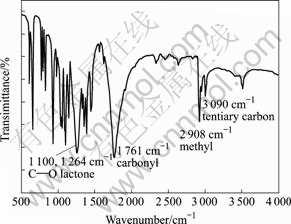
Fig.3 IR spectrum of lactide crystals
Table 2 Optical activity of lactide catalyzed by different catalysts

3.6 Morphology of lactide
Fig.5(a) shows the SEM image of the cross section of lactide crystals catalyzed by ZnO; it presents a regular dendriform, and the edge is concave. Fig.5(b) shows the SEM image of the cross section of lactide crystals catalyzed by nanocrystals of La-Ti composite oxides; it presents a feature of perforated fracture with pores embedded. By comparision of Fig.5(a) with (b), it can be learnt that the crystal planes of the lactides catalyzed by ZnO and nanocrystals of La-Ti composite oxides are different and the grain-boundary strength of the latter is stronger than that of the former.
3.7 Discussion
Generally, for the purpose of preparing D-, or L-lactide, D-, or L-lactic acid is usually used as the raw material. However, herein, chiral lactides were obtained with D-, L-lactic acid catalyzed by nanocrystals of La-Ti composite oxides. The appearance of chirality is due to the microphase separation or the change of the ratio of D-lactide to L-lactide. It can be attributed to the unique catalytic activity of the catalysts. It is well known that there are many hollow orbits (La 4f 05d16s2) in the outer electron structure of lanthanum. And the composition of titanium oxide will generate numerous oxygen vacancies. Consequently, the catalysts can selectively combine with D-, L-lactic acid to form chiral complex. This may lead to the change of the ratio of L-lactic acid to D-lactic acid in the oligomers. And as a result, the splitting of the oligomers will lead to different ratios of D-lactide to L-lactide. Actually, it is necessary to study the formation process of oligomers to confirm the above reasoning.

Fig.4 Micropolariscope photos of lactides catalyzed by La-Ti composite oxides in different solvents: (a) Ethanol as solvent; (b) Acetic acid as solvent

Fig.5 SEM images of D-, L- lactides catalyzed by different catalyst: (a) ZnO as catalyst; (b) La-Ti composite oxide as catalyst
4 Conclusions
1) The La-Ti composite oxides with a narrow and homogeneous particle size distribution are prepared by sol-gel method.
2) The activity of nanocrystals of La-Ti composite oxides prepared via a sol-gel route towards the catalysis of D-, L-lactic acid to prepare lactide is studied. The results demonstrate that the ratio of D-lactide to L-lactide is not equal to 1?1, which suggests that micro phase separation might occur in lactide crystals.
3) The as-prepared nanocrystals of La-Ti composite oxides may be potential catalysts with a good selectivity.
References
[1] ZHOU Zhong-cheng, RUAN Jian-ming, HUANG Bai-yun, LI Ya-jun, ZOU Jian-peng, ZHANG Hai-bo. Preparation and characterization of poly(D, L-lactide) and its porous biomaterials [J]. Journal of Central South University of Technology, 2005, 12(1): 1-4.
[2] WANG Chen-hong, LI Hong, ZHAO Xiao-na. Ring opening polymerization of L-lactide initiated by creatinine[J]. Biomaterials, 2004, 25(27): 5797-5801.
[3] KULKARNI R K, PANI K C, NEUMAN C, LEONARD F. Polylactic acid for surgical implants [J]. Archive Surgery, 1966, 93: 839-843.
[4] RUAN Jian-ming, ZOU Jian-peng, HUANG Bai-yun. Biomaterial science [M]. Beijing: Science Press, 2004. (in Chinese)
[5] MASAKI N, HISASHI O. Manufacture of high pure lactides by depolymerization of lactic acid oligomers and catalysts therefore: Japan, 5310782 [ P]. 2000-05-26.
[6] STEVEN-COHEN R M D, PAUL-MITTERMILLER A, ALPH- HOLMES R E M D, KEVIN BRODER W M D. Clinical experience with a new fast-resorbing polymer for bone stabilization in craniofacial surgery [J]. Journal of Craniofacial Surgery, 2006, 17(1): 40-43.
[7] BETTINGER C J, ORRICK B, MISRA A, LANGER R, BORENSTEIN J T. Microfabrication of poly (glycerol-sebacate) for contact guidance applications [J]. Biomaterials, 2006, 27 (12): 2558-2565.
[8] OHKITA T, LEE S H. Thermal degradation and biodegradability of poly (lactic acid)/corn starch biocomposites [J]. Journal of Applied Polymer Science, 2006, 100 (4): 3009-3017.
[9] CHENG Chao, WANG Yuan-liang. The progress of catalysts for preparation of lactide [J]. Chemical Research and Application, 2007, 19(4): 358-360. (in Chinese)
[10] LIANG Bao-xia, LIU Peng-sheng. Screen of catalysts for preparation of D-, L-lactide [J]. Journal of Xiangtan University: Science Edition, 2004, 26(1): 59-70. (in Chinese)
[11] GU Li, WANG Yuan-liang, YANG Hua, TANG Li-ling, XIA Lie-wen. Surface topography of La-Ti composite oxide nanocrystallines examined with atomic force microscope [J]. Trans Nonferrous Met Soc China, 2003, 13(2): 294-297.
[12] GU Li, WANG Yuan-liang, ZHU Jiu-jin, XIA Lie-wen. The preparation of ultra-pure D, L-lactide catalyzed by nanocrystalline La-Ti composite oxide [C]// Proceedings of International Congress on Biological and Medical Engineering. Singapore: National University of Singapore, 2002: 12-13.
[13] LI Cao, WANG Yuan-liang. Solid super strong acid of SO42-/ZnO-SnO2/La3+ act as catalyst for preparation of lactide [J]. Chemistry and Adhesion, 2003(1): 32-36. (in Chinese)
[14] SU Tao, XIN Yu-ying, MA Hua. Preparation of D, L-lactide by air flow of CO2 under ordinal pressure [J]. Fine Chemical Engineering, 1999, 16(3): 41-43. (in Chinese)
[15] LI Xiao-hong, XIONG Cheng-dong. Preparation of poly lactic acid, its copolymer and their application in biomedical science [J]. Macromolecular Bulletin, 1999(1): 24-32. (in Chinese)
[16] LUO Bing-hong, ZHOU Chang-run. Preparation and characterization of monomer for functional poly lactic acid [J]. Acta Scientiarum Nuturalium Universitatis Sunyatseni, 2005, 44(3): 67-70. (in Chinese)
[17] GU Li, WANG Yuan-liang, ZHU Jiu-jin, WU Jun-han. Preparation of La-Ti composite oxide nanocrystal and examination of their surface topography with atomic force microscope [J]. Chinese Chemistry Letters, 2003, 14(4): 429-432.
[18] GU Li. The preparation of composite oxides nanocrystalline and its catalysis for synthesis of lactide and poly lactic acid [D]. Chongqing: Chongqing University, 2002: 45-53. (in Chinese)
Foundation item: Project(50174059) supported by the National Natural Science Foundation of China; Project(Y406469) supported by Natural Science Foundation of Zhejiang Province
Received date: 2008-03-20; Accepted date: 2008-05-20
Corresponding author: GU Li, PhD, Associate professor; Tel: +86-573-83646195; E-mail: guli@mail.zjxu.edu.cn
(Edited by YANG Hua)