
Microwave absorbing mechanism of soft magnetic alloy Fe85Si2Al6Cr7
WANG Zhong-you(王忠友), JIANG Jian-jun(江建军), DENG Lian-wen(邓联文),
ZHANG Chuan-kun(张传坤), ZHANG Tao-qi(张韬奇)
Department of Electronic Science and Technology, Huazhong University of Science and Technology,Wuhan 430074, China
Received 15 July 2007; accepted 10 September 2007
Abstract: The powders of amorphous nanocrystalline Fe85Si2Al6Cr7 were prepared by high energy ball milling for different times, and measured by XRD and network analyzer. The results show that: 1) nanocrystalline microstructure remarkably improves the microwave permeability, and the permittivity is controlled effectively; 2) by adding proper dyeing auxiliary (such as copper phthalocyanine), the magnetic properties of powders are improved when the particle sizes milled are excessively small.
Key words: microwave absorbing mechanism; complex permeability; complex permittivity; ball-milling time
1 Introduction
With the high development of wireless communication, EM wave absorbing materials continue to attract much attention as EM interference-suppressing coatings, self-concealing technology and microwave darkrooms. Among the candidates for such applications, the soft metallic magnetic materials are a promising and interesting set of materials. Since metallic magnetic materials have large saturation magnetization and the Snoek’s limit is at a high frequency[1-4], their complex permeability values remain high over a wide frequency range. Therefore, it is possible to make thin absorbers from such kinds of materials.
The high performance absorbers need materials of high-permeability. For improving permeability, the particles of absorber have become fine[5], nano- crystalline grain multiphase[6-7], and particles flatted [8-9]. Amorphous nanocrystalline alloys FeSiAlCr are excellent soft magnetic materials with wideband absorbing[10]. In this article, we report the electro- magnetic dynamic properties of Fe85Si2Al6Cr7, and the method of improving its microwave performance.
2 Experimental
Amorphous nanocrystalline alloys Fe85Si2Al6Cr7 (mole fraction, %) of rapid-quench ribbon were put into stainless steel jar of XPWL high-energy ball-miller with steel-ball after it had been cut into fragments. The ratio of ball to materials was 20?1, the speed was 450 r/min, and acetone was process control agent(PCA). sample- powder was taken separately at 3, 6, 9, 12, 15 and 18 h after the materials were milled to analyze structure and measure magnetic parameter.
The patterns of powder were characterized by scanning electron microscopy(SEM). The powder was also characterized by powder X-ray diffraction(XRD), using Cu Kα radiation (λ=0.154 178 nm). The grain size was computed by Scherrer’s formula. These powders were mixed with a paraffin at a ratio of 80% (mass fraction) (powder) and then formed into toroidally shaped samples (Φout=7.00 mm, Φin=3.04 mm, t=3.0-3.60 mm). The measurements of permeability and permittivity of the samples were carried out using an Aligen HP8722 vector ES S-parameter network analyzer in the 2-18 GHz range.
3 Results and discussion
3.1 Structural characteristics and analysis
Fig.1 shows the XRD patterns of the alloys before and after ball-milling. Before ball milling, there were much narrow X-ray diffractive-peaks. This phenomenon indicates that a few of diffractive-peaks of other grains
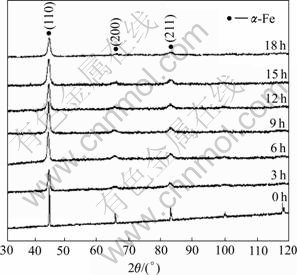
Fig.1 XRD patterns of amorphous nanocrystalline Fe85Si2Al6- Cr7 sample milled for different times
(such as FeSi3) exist, besides diffractive-peak of α-Fe. But, after ball milling, the number of XRD diffractive-peaks of α-Fe decreased, and the diffractive-peaks of α-Fe were broadened and fell obviously. This causation is: the particles of alloys were struck strongly by steel balls in the steel-jar, particle sizes decreased, and the defects of α-Fe grain increased with increasing ball-milling time. The ferreous lattice structure is α-Fe all the time without new phase after ball-milling, but the ferreous interplanar spacing has a disciplinarian change with the ball-milling time. Table 1 lists the variational rule of lattice spacing of the samples with milling time along [110], [200] and [211] lattice-directions. The lattice strain along [110] is larger than those along [200] and [211] with milling time from 3 h to 18 h, and referring to Table 1. The reason for the phenomenon is that the anisotropy of material reduces obviously with the ball-milling time, and it’s similar to the results of reported by WANG et al[11].
Table 1 Variations of lattice spacings of α-Fe in Fe85Si2Al6Cr7 magnetic particles with milling time

The average grain sizes of the sample can be estimated by Scherrer’s formula:
 (1)
(1)
where D is the grain size, λ is the wavelength of the X-rays, θ is the diffraction angle and β is the FWHM of the peak. The average grain sizes are listed in Table 2. Obviously, the average grain size decreases with the ball-milling time increasing, and approaches to 10 nm.
Table 2 Intrinsic grain sizes of Fe85Si2Al6Cr7 after different ball-milling time

As shown in Fig.2, the powders of Fe85Si2Al6Cr7 with flake-like particles were obtained after 3h ball-milling. With the milling time increasing, the size and thickness of flake particles have already been refined to submicrometer, and the aspect ratio of particle is about 10.
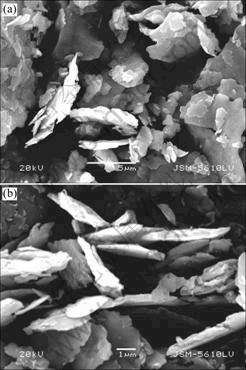
Fig.2 SEM images of Fe85Si2Al6Cr7 ball-milled for 9 h (a) and 15 h (b)
3.2 Microwave electromagnetic parameters
Figs.3 and 4 show the electromagnetic spectra of coaxial samples measured by vector network analyzer.
Fig.3 shows the complex permeability of the samples. The real part of complex permeability increases

Fig.3 Complex permeability after different milling times: (a) Real permeability; (b) Imaginary permeability
with milling time increasing in Fig.3(a). Because the smaller average grain sizes of powders were gotten with ball-milling time increasing, then the effective anisotropy would reduce, according to famous Herzer’s model[12], and the permeability was enhanced. This model predicted that the effective anisotropy(K) varied as the six-order of the grain size(D) in the range D<Lex (Lex denotes the ferromagnetic exchange length), that is K= where K1 and A are the magnetocrystalline anisotropy constant and the exchange stiffness constant, respectively. By the uniform rotational magnetization mechanism and Hezer’s result, we can also get the relationship of the coercive force Hc and permeability μi in connection with the grain diameter D, which are Hc?D6, μi?D-6, respectively. Obviously, the average grain sizes of the samples satisfied the condition D<Lex (for α-Fe, A=10-11 L/m, K1=8 kJ/m3, then Lex= (A/K)1/2=35 nm). So the real permeability was improved with prolonging of the ball-milling time from 3 h to 15 h. But the magnetic properties would become
where K1 and A are the magnetocrystalline anisotropy constant and the exchange stiffness constant, respectively. By the uniform rotational magnetization mechanism and Hezer’s result, we can also get the relationship of the coercive force Hc and permeability μi in connection with the grain diameter D, which are Hc?D6, μi?D-6, respectively. Obviously, the average grain sizes of the samples satisfied the condition D<Lex (for α-Fe, A=10-11 L/m, K1=8 kJ/m3, then Lex= (A/K)1/2=35 nm). So the real permeability was improved with prolonging of the ball-milling time from 3 h to 15 h. But the magnetic properties would become
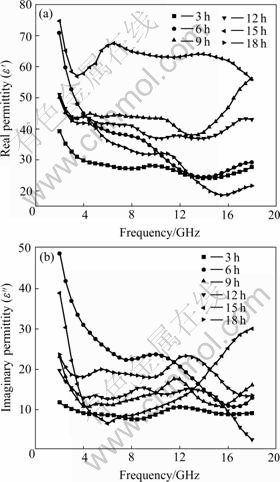
Fig.4 Complex permittivity after different milling times: (a) Real permittivity; (b) Imaginary permittivity
bad when the ball-milling time is too long (such as 18 h), because of the magnetocrystalline anisotropy constant K1 increased (refer to Table 1).
Fig.3(b) shows the spectrum of the imaginary part of permeability with ball-milling time. With increasing ball-milling time, the imaginary part increases. The resonance peaks become more obvious after 9 h, and move to the higher frequency. This may be caused by the size-effect, reduced anisotropy and flat-shape effect of the particle[13-15].
The spectra of the complex permittivity of the samples after different milling-times are shown in Fig.4. The complex permittivity of the original materials is low, but the complex permittivity after ball-milling increases obviously with ball-milling time increasing. The reason is possibly enhanced polarization on surface of particles, because the number of atoms on the surface of particles increases, the specific areas and the surface activity of particles are advanced with increasing ball-milling time.
3.3 Comparison of electromagnetic performance for alloys before and after adding dyeing auxiliary
According the above discussion, when the milling time reaches 18 h, the complex permeability decreases. The reason is possibly that the number of bare atoms on the particle surface are added when the particles are refined excessively, the activity of particles increases, the surfaces of particles are oxidated, so the electromagnetic performance is bad. In order to test this guess, the samples of the same materials were milled after adding the dyes of copper phthalocyanine (1.4 mg in 100 g materials), and the acetone was used as PCA, and milling-time was 18 h, then the coaxial sample was made. The sample was measured by vector network analyzer, its electromagnetic curve is shown in Fig.5. The electromagnetic performance of the sample is improved much after adding copper phthalocyanine. The reason is that the oxidation degrees of the surface atoms of the particles are decreased by adding copper phthalocyanine [16].

Fig.5 Variations of complex permeability before and after adding copper phthalocyanine
3.4 Simulation computing of reflectivity curve
For monolayer absorbing materials of thickness d, the absorber’s reflection coefficient is (in dB ):
 (2)
(2)
where μ0 and ε0 are the permeability and permittivity respectively in vacum condition; μr and εr are the complex permeability and complex permittivity of magnetic materials respectively; d is the thickness of absorbing materials; f is the frequency.
According to formula (2), the measured data of electromagnetic parameter were input, then the reflectivity of absorbing materials about 1 mm thickness were calculated out in the range of 2-18 GHz. Its curve is shown in Fig.6. It shows that the absorbing bandwidth is wider at 3 h, the absorbing values move to low- frequency with milling-time delayed from 3 h to 15 h, and the microwave absorbing capability of low- frequency is good, but the capability at 18 h falls without adding copper phthalocyanine.

Fig.6 Energetic loss of magnetic reflect about 1 mm thickness after different milling times
4 Conclusions
1) Nanocrystalline microstructure remarkably improves the microwave permeability and the permittivity is controlled effectively.
2) After adding proper dyeing auxiliary (such as phthalocyanine-copper), the degree of oxidation of particle is reduced, and the magnetic properties are improved.
References
[1] FERGEN I, SEEMANN K, WETH A V D, SCH?PPEN A. Soft ferromagnetic thin films for high frequency applications [J]. J Magn Magn Mater, 2002, 242/245: 146-151.
[2] YOSHIDA S, SATO M, SUGAWARA E, SHIMADA Y. Permeability and electromagnetic-interference characteristics of Fe-Si-Al alloy flakes-polymer composite [J]. J Appl Phys, 1999, 85(8): 4636-4638.
[3] DENG L W, JIANG J J, FAN S C, FENG Z K, XIE W Y, ZHANG X C, HE H H. GHz microwave permeability of CoFeZr amorphous materials synthesized by two-stepmechanical alloying [J]. J Magn Magn Mater, 2003, 264: 50-54.
[4] SNOCK J L. Dispersion and absorption in magnetic ferrites at frequencies above one Mc/s [J]. Physica, 1948, 14(4): 207-217.
[5] WU L Z, DING J, JIANG H B, CHEN L F, ONG C K. Particle size influence to the microwave properties of iron based magnetic particulate composites [J]. J Magn Magn Mate, 2005, 285(1/2): 233-239.
[6] HERNANDO A, VAZQUEZ M, KULIK T, PRADOS C. Analysis of the dependence of spin-spin correlations on the thermal treatment of nanocrystalline materials [J]. Phys Rev B, 1995, 51(6): 3581-3586.
[7] HASHIN Z, SHTRIKMAN S. A variational approach to the theory of the effective magnetic permeability of multiphase materials [J]. J Appl Phys, 1962, 33(10): 3125-3131.
[8] ALVES F, RAMIARINJAONA C, B?RENGUER S, LEBOURGEOIS R, WAECKERL? T. High-frequency behavior of magnetic composites based on FeSiBCuNb particles for power electronics [J]. IEEE Trans Magn, 2002, 38(5): 3135-3137.
[9] MAZALEYRAT F, LEGER V, LEBOURGEOIS R, BARRUE R. Pereability of soft magnetic composites from flakes of nanocrystalline ribbon [J]. IEEE Trans Magn, 2002, 38(5): 3132-3134.
[10] JIANG J J, FU Q W, DENG L W, HE H H. Microwave absorbing capability of nanocrystalline flakes of Fe85SilAl6Cr8 [J]. J Huazhong Univ of Sci & Tech (Nature Science Edition), 2006, 34(12): 89-91. (in Chinese)
[11] WANG W, GUAN J G , WANG Q. Influence of ball milling time on the microstructure and properties of prepared Fe-ZnO core-shell nanocomposite particles [J]. J Inor Mater, 2005, 20(3): 599-607. (in Chinese)
[12] HERZER G. Grain structure and magnetism of nanocrystalline ferromagnets [J]. IEEE Trans Magn, 1989, 25(5): 3327-3329.
[13] CHIN T S, HUANG S H, YAU J M. Anisotropic Nd-Fe-M-B flakes by single-roller melt spinning [J]. Appl Phys Lett, 1991, 59(16): 2046-2048.
[14] YOSHIDA S, ANDO S, SHIMADA Y, SUZUKI K, NOMURA K, FUKAMICHI K. Crystal structure and microwave permeability of very thin Fe-Si-Al flakes produced by microforging [J]. J Appl Phys, 2003, 93: 6659-6661.
[15] KIM S S, KIM S T, YOON Y C, LEE K S. Magnetic, dielectric, and microwave absorbing properties of iron particles dispersed in rubber matrix in gigahertz frequencies [J]. J Appl Phys, 2005, 97: 10F905-1-3.
[16] ZHAO P, LI Y, LIANG Q. Inhibition effect of metal-free phthalocyanine, copper phthalocyanine and zinc phthalocyanine on mild steel in 1 mol/L H2SO4 [J]. Corro Sci & Prot Techn, 2006, 18(4): 235-240. (in Chinese)
(Edited by PENG Chao-qun)
Foundation item: Project(50371029) supported by the National Natural Science Foundation of China
Corresponding author: JIANG Jian-jun; Tel: +86-27-87544472; E-mail: jiangjj@mail.hust.edu.cn