J. Cent. South Univ. Technol. (2010) 17: 467-471
DOI: 10.1007/s11771-010-0508-2 
Preparation of poly(amino-quinone) by microwave-assisted
solid-state polymerization
LI Hai-pu(李海普), WAN Jun-jie(万俊杰), WANG Shuai(王帅), CHANG Qing-wei(常庆伟)
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2010
Abstract: Microwave irradiation was employed to assist the synthesis of poly(amino-quinone) (PAQ) from p-benzoquinone and diamines in solid state. The effects of power, time, and pattern (continuously or intermittently) of microwave irradiation on yield and intrinsic viscosity of PAQs were studied. It is shown that the continuous microwave irradiation at a high power leads to rapid increase of yield and a sudden halt in polymerization afterwards, due to the subsequent loss of volatile reactants at a high reaction temperature. Alternatively, the high-power microwave irradiation is applicable to raising the yield if used intermittently. In contrast, the low-power microwave irradiation favours the way of continuous exposure to ensure sufficient heat for polymerization. In both cases of high and low power, the yield and intrinsic viscosity can be further promoted by prolonging the exposure time. It is found that under a preliminarily optimized condition of intermittent irradiation at 490 W with six sequences of 5 min irradiation followed by 5 min interval, the yield and intrinsic viscosity of PAQ from p-benzoquinone and p-phenylene diamine can reach as high as 83% and 41.9 mL/g, respectively.
Key words: poly(amino-quinone); microwave; solid-state reaction
1 Introduction
Since the work of KALEEM et al [1], poly(amino- quinone) (PAQ) has been recognized as an efficient polymer material for coatings [2] and adhesives [3] because of its extraordinary metal-affinity [4], good thermal resistance [5], impact strength [6], and reversible redox property [7]. PAQ has also found interesting applications as a binder in the prevention of polymer scale deposition [8] and as a supporter in the assembly of advanced functional materials [9].
The majority of previously reported PAQs was prepared by the reaction of amines with quinone in solution, with an emphasis on the selection of oxidizing agents [10] and substitutes on amines [11] in the hope of improving the efficiency of reaction as well as solubility and stability of polymer [12]. The main disadvantage of solution polymerization is the waste of large quantities of organic solvent and hence the environment pollution. Compared with the conventional solution chemistry, solid-state synthesis is more desirable due to simplification of reaction work and product isolation. Surprisingly, the synthesis of PAQ by solid-state polymerization is scarce, possibly due to the low yield [13]. Accordingly, raising the yield of PAQ by solid-state reaction becomes an attractive and challenging subject.
Recently, microwave-assisted synthesis has becom a frontline methodology and gained growing attention in organic [14-16], medicinal [17-19], and polymer [20-23] chemistry. It especially facilitated the solid-state reactions in view of facile acceleration, improved yield [24-26], and green concept [27-28]. Additionally, the effects of microwave irradiation on polymer properties were also reported in various syntheses [29-33]. However, to the best of our knowledge, there has been no report on microwave-assisted solid-state synthesis of PAQ in documents. Thus, in this work, the introduction of microwave irradiation to the synthesis of PAQ in solid state was reported, and the effects of microwave power, irradiation time and pattern on the yield and intrinsic viscosity of the obtained polymers were investigated.
2 Experimental
2.1 Polymerization
The mixture of analytical grade p-benzoquinone (PBQ) and o-phenylene diamine (o-PDA) or p-phenylene diamine (p-PDA) was manually ground in an agate mortar for 10 min, and then transferred into a microwave oven. The reactants were radiated at different microwave powers, such as 70, 210, 350, 490 and 700 W, using various time profiles. The resulting product was dissolved in N, N-dimethyl formamide (DMF) and then precipitated in water, followed by filtration and repeated wash with hot water.
2.2 Measurement
The fully dried product was weighed, and the yield of PAQ was calculated according to the following equation:
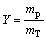 ×100% (1)
×100% (1)
where mp is the weight of product, g; mT is the theoretical weight of polymer, g.
The intrinsic viscosity [η] of PAQ was determined by Eqs.(2)-(4).
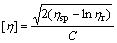 (2)
(2)
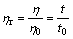 (3)
(3)
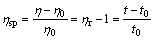 (4)
(4)
where C represents the concentration of PAQ in DMF solution; t0 and t correspond to the flow times of the pure solvent of DMF and the polymer solution (2 g/L), respectively, which are measured at 25 ℃ on an Ubbelohde viscometer with the capillary diameter of 0.38 mm; η0 and η denote the viscosities of DMF and PAQ-DMF solution, respectively; ηr and ηsp refer to the relative viscosity and specific viscosity, respectively.
2.3 Characterization
The synthesized PAQs were characterized by FTIR (AVATAR360, Nicolet, USA) and UV-Vis (756 MC, Shanghai, China) spectroscopy.
3 Results and discussion
Microwave heating has proved to be a very promising non-conventional tool for solid-state synthesis. In the synthesis of PAQ from PBD and o-PDA, microwave irradiation is tentatively employed to assist the solid-state reaction. As depicted in Fig.1, the UV-Vis spectrum of the obtained products exhibits a characteristic peak at 349 nm, confirming the overwhelming presence of disubstituted benzoquinone [4]. Likewise, FTIR spectra [34] are featured by typical adsorption bands for —NH— (3 200-3 250 cm-1) and  (1 640-1 650 cm-1) functional groups, which are quite consistent with the previous reports on a wealth of PAQs [4, 12, 35]. These results by solid-state polymerization also well correspond to those obtained in products synthesized by solution polymerization done in our laboratory [34].
(1 640-1 650 cm-1) functional groups, which are quite consistent with the previous reports on a wealth of PAQs [4, 12, 35]. These results by solid-state polymerization also well correspond to those obtained in products synthesized by solution polymerization done in our laboratory [34].
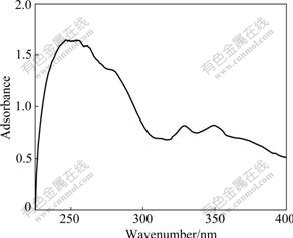
Fig.1 UV-Vis spectrum of PAQ
Furthermore, the influences of microwave power on the yield of synthesized products were examined. As shown in Fig.2(a), the yields of resultant products are found to increase with the increase of microwave power except for the series at 700 W. In all cases, the yields exceed 20% after 15 min and then reach nearly 40% within 30 min, which is in sharp contrast to the yield of 16% obtained in a parallel solid-state reaction lasting for 30 min in the absence of microwave irradiation. At the same time, Fig.2(a) also exhibits that the irradiation time is another key factor for the solid-state polymerization, since a lengthy irradiation time would result in an increase in yield of PAQ. These results indicate that microwave-assisted solid-state polymerization leads to dramatic reduction in reaction time, benefiting from the efficient internal heating by direct coupling of microwave energy with the polar moiety in the reaction mixtures [17].
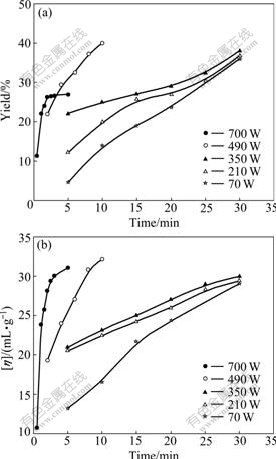
Fig.2 Effects of microwave power and irradiation time on yield (a) and [η] (b) by continuous exposure
Likewise, a microwave power dependence of intrinsic viscosity is observed in Fig.2(b). The value of [η] surpasses 20 mL/g within 10 min when the microwave power locates in the range of 210-700 W, while it takes 15 min at a low microwave power of 70 W. To make a further comparison, a reference reaction of PBQ with an equivalent of o-PDA was carried out in THF solution. It turns out to take 3 h at 70 ℃ to render the product with [η] of 18.27 mL/g via solution polymerization. These results strongly suggest that the microwave heating is more efficient to raise [η] of PAQ than conventional solution polymerization.
A bundle of practices have proved that microwave irradiation was facile to render rapid and efficient syntheses of polymers with high viscosity, such as polyamide [36], poly(amide-imide) [37-38], poly(ester- imide) [39], poly(meth)acrylamide [40], and poly(amic- acid) [41]. However, divergent observations did occur, though sparsely [32, 42]. Accordingly, the influence of microwave irradiation on the intrinsic viscosity of polymers is complicated, so that the general rule that can be safely adopted in each specific case is still pending.
From Fig.2(a), it is impressively observed that at an intense microwave power of 700 W the yield of PAQ undergoes a rapid growth within the initial 2 min and then retardedly increases at the level of 26% when further treated with microwave irradiation. This deviation could be ascribed to the excessive microwave irradiation, which drastically raised the reaction temperature and hence hastened the loss of volatile components in reactants. To make a further investigation, the variation of reaction temperature with time was monitored at determined microwave powers (Fig.3(a)). It can be clearly seen that a continuous irradiation at 700 W leads to a sharp increase in reaction temperature above 90 ℃ within 5 min, while a lower microwave power corresponds to a milder increase in reaction temperature.
To practically utilize the efficiency of the high microwave power and discourage the rapid accumulation of energy in the meantime, a strategy of intermittent irradiation was proposed for the current system. As shown in Fig.3(b), in both cases of 490 W and 350 W the time for reaching 90 ℃ by intermittent exposure is roughly 0.5 times prolonged when compared with that by continuous exposure. However, under the same condition there is less improvement at 700 W. These observations suggest that the high microwave power in proper range could afford better results by working intermittently in lengthy time. As for the power as high as 700 W, the pulsed microwave could possibly be a solution [43].
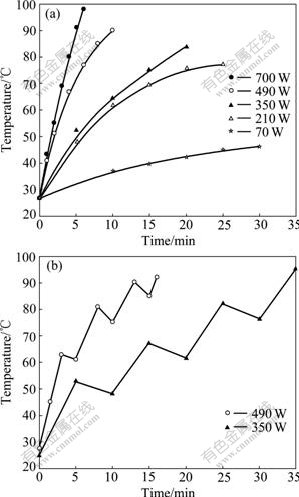
Fig.3 Reaction temperature with time by continuous (a) and intermittent (b) exposure
Fig.4(a) shows the results obtained at 350 W by intermittent exposure. As expected, the intermittent irradiation at high power simply results in a noticeable increase in the yield of PAQ relative to that by continuous exposure. At the same time, it was found that a rational sequence of intermittent irradiation could further improve the results with a constant overall irradiation time. Additionally, we are also interested in whether the intermittent working pattern is applicable to low microwave power. As illustrated in Fig.4(b), however, the situation is changed in the case of low microwave power of 70 W. The intermittent irradiation at 70 W causes a gradual drop in yields of PAQ when compared with that by continuous exposure, which could be attributable to the insufficient accumulation of heat. Moreover, it is noted that the irradiation, employed intermittently or continuously at high power (350 W) or low power (70 W), has few influence on [η] of PAQ, as long as the overall irradiation time in respective case is kept the same.
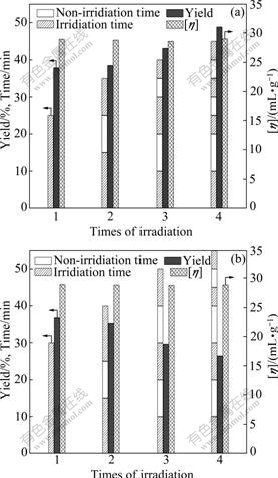
Fig.4 Effects of intermittent irradiation on yield and [η] at 350 W (a) and 70 W (b)
According to the observations above, it could be concluded that the intermittent irradiation at high microwave power (350 W and 490 W) with rationally designed sequences allows for easier temperature control and hopefully paves a way for giving PAQs with both higher yield and [η] by solid-state polymerization.
To further explore the potential of high power microwave irradiation, six sequences composed of 5 min irradiation followed by 5 min interval with extra grinding therein were applied to the solid-state synthesis of PAQ from PBD and p-PDA at 350 W and 490 W. As shown in Fig.5, the yield and [η] of PAQ gain further improvement. Especially, within 60 min at 490 W, the yield and [η] of PAQ reach as high as 83% and 41.9 mL/g, respectively.
4 Conclusions
(1) The solid-state synthesis of PAQ is greatly influenced by microwave irradiation, and this effect is complicated and varies with respect to microwave power.
(2) Generally, in the proper range of microwave power (70-490 W), higher power irradiation by continuous exposure corresponds to higher yield and intrinsic viscosity of PAQ, which could be further promoted with lengthy reaction time.
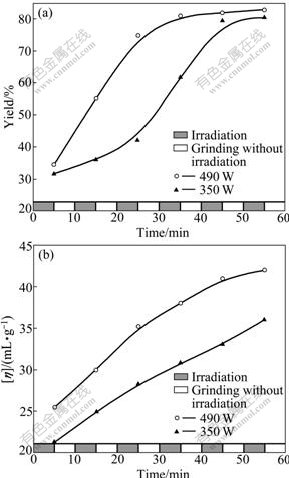
Fig.5 Yield (a) and [η] (b) with time by intermittent irradiation at high microwave power
(3) At high microwave power (350 and 490 W), intermittent irradiation with rationally designed sequences provides a facile way to raise both the yield and intrinsic viscosity of PAQ by solid-state synthesis.
References
[1] KALEEM K, CHERTOK F, ERHAN S. A novel coating based on poly(etheramine-quinone) polymers [J]. Progress in Organic Coatings, 1987, 15: 63-71.
[2] HAN M J, HELMS A B, HU Y Q, NIKLES D E. Amine-quinone polymers and the protection of iron particles against corrosion [J]. IEEE Transactions on Magnetics, 1999, 35(5): 2763-2765.
[3] JIN F L, HAN M, PARK S J. Fracture and adhesion behaviors of epoxy resins modified with poly(amine-quinone) [J]. Polymer International, 2006, 55(11): 1265-1269.
[4] JEYAPRABHA C, SATHIYANARAYANAN S, PHANI K L N, VENKATACHARI G. Influence of poly (aminoquinone) on corrosion inhibition of iron in acid media [J]. Applied Surface Science, 2005, 252(4): 966-975.
[5] REDDY T A, MACAIONE D, ERHAN S. Quinone-amine polymers: ⅩⅤ. Syntheses and characterization of high-temperature resistant poly(arylamino-quinone)s [J]. Journal of Polymer Science, Part A: Polymer Chemistry, 1994, 32(10): 1977-1982.
[6] NITHIANANDAM V S, ERHAN S. Quinone-amine polymers: ⅩⅧ . A novel method for the synthesis of poly(alkyl aminoquinone)s [J]. Polymer, 1998, 39(17): 4095-4098.
[7] MASIUKIEWICZ A W. Electron super-exchange mechanism and the residual mobility of topa quinone in amine oxidases [J]. Abstracts of Papers of the American Chemical Society, National Meeting, 2001, 222: U228-U228.
[8] KOMABASHIRI T, TSUJINAKA M, MITANI T. Method of preventing polymer scale deposition: US 4970278 [P]. 1990-11-13.
[9] LIANG J L, NIKLES D E. Amine-quinone polyurethanes as binders for metal particle tape [J]. IEEE Transactions on Magnetics, 1993, 29(6): 3649-3651.
[10] NITHIANANDAM V S, ERHAN S. Quinone amine polymers: Ⅸ. Attempts to synthesize polyamine-enzoquinone polymers using air and oxygen as oxidizing agents [J]. Journal of Applied Polymer Science, 1991, 42(9): 2385-2389.
[11] DIBATTISTA J, SCHMIDT B M, PADIAS A B, HALL H K. Substituent effects on the polycondensation of quinones with aromatic amines to form poly(quinone imine)s [J]. Journal of Polymer Science, Part A: Polymer Chemistry, 2002, 40(1): 43-54.
[12] REDDY T A, ERHAN S. Quinone-amine polymers: Ⅻ. Synthesis and characterization of novel polymers from 2-phenylbenzoquinone and aliphatic diamines [J]. Journal of Polymer Science, Part A: Polymer Chemistry, 1994, 32(3): 557-565.
[13] SINGH N B, SINGH R J. Solid-state reaction between p-phenylenediamine and p-benzoquinone [J]. Journal of Solid State Chemistry, 1988, 76: 375-390.
[14] LIN M, ZHOU J M, XIA H P, YANG R F, LIN C. Microwave irradiation promoted synthesis of aryloxy acetic acids [J]. Chemical Research in Chinese Universities, 2004, 20(2): 213-215.
[15] LI M, ZHANG Q J, YANG J W, TONG S L, YANG K E, XIAO F S, DENNIS K P N, YAN Y. An efficient and simple reaction: Solvent- free preparation of rosolic acid under microwave irradiation [J]. Chemical Research in Chinese Universities, 2008, 24(3): 385-388.
[16] HOOGENBOOM R, WILMS T F A, ERDMENGER T, SCHUBERT U S. Microwave-assisted chemistry: A closer look at heating efficiency [J]. Australian Journal of Chemistry, 2009, 62(3): 236- 243.
[17] KAPPE C O, DALLINGER D. The impact of microwave synthesis on drug discovery [J]. Nature Reviews Drug Discovery, 2005, 5(1): 51-63.
[18] KAPPE C O, LARHED M. All the rave in microwaves [J]. Angewandte Chemie International Edition, 2005, 44(47): 7666- 7669.
[19] WANNBERG J, ERSMARK K, LARHED M. Microwave- accelerated synthesis of protease inhibitors [J]. Topics in Current Chemistry, 2006, 266: 167-198.
[20] VOUYIOUKA S N, KARAKATSANI E K, PAPASPYRIDES C D. Solid state polymerization [J]. Progress in Polymer Science, 2005, 30(1): 10-37.
[21] LIU L, LI Y, LI Y, FANG Y E. Rapid N-phthaloylation of chitosan by microwave irradiation [J]. Carbohydrate Polymers, 2004, 57(1): 97- 100.
[22] LOBERT M, KOHN U, HOOGENBOOM R, SCHUBERT U S. Synthesis and microwave assisted polymerization of fluorinated 2-phenyl-2-oxazolines: The fastest 2-oxazoline monomer to date [J]. Chemical Communications, 2008(12): 1458-1460.
[23] HOOGENBOOM R, SCHUBERT U S. Microwave-assisted organic and polymer chemistry [J]. Australian Journal of Chemistry, 2009, 62(3): 181-183.
[24] NAVALADIAN S, VISWANATHAN B, VARADARAJAN T K, VISWANATH R P. Microwave-assisted rapid synthesis of anisotropic Ag nanoparticles by solid state transformation [J]. Nanotechnology, 2008, 19: 45603-45609.
[25] COANTIC S, SUBRA G, MARTINEZ J. Microwave-assisted solid phase peptide synthesis on high loaded resins [J]. International Journal of Peptide Research and Therapeutics, 2008, 14(2): 143-147.
[26] DIAZ-MOCHON J J, FARA M A, SANCHEZ-MARTIN R M, BRADLEY M. Peptoid dendrimers-microwave-assisted solid-phase synthesis and transfection agent evaluation [J]. Tetrahedron Letters, 2008, 49: 923-926.
[27] WIESBROCK F, HOOGENBOOM R, SCHUBERT U S. Microwave-assisted polymer synthesis: State-of-the-art and future perspectives [J]. Macromolecular Rapid Communications, 2004, 25(20): 1739-1764.
[28] STRAUSS C R, VARMA R S. Microwaves in green and sustainable chemistry [J]. Microwave Methods in Organic Synthesis, 2006, 266: 199-231.
[29] HOOGENBOOM R, LEENEN M A M, HUANG H Y, FUSTIN C A, GOHY J F, SCHUBERT U S. Microwave-assisted synthesis and micellization behavior of soy-based copoly(2-oxazoline)s [J]. Colloid and Polymer Science, 2006, 284(11): 1313-1318.
[30] HOOGENBOOM R, SCHUBERT U S. Microwave-assisted polymer synthesis: Recent developments in a rapidly expanding field of research [J]. Macromolecular Rapid Communications, 2007, 28(4): 368-386.
[31] LEE S J, SANDHU K S, LIM S T. Effect of microwave irradiation on crystallinity and pasting viscosity of corn starches different in amylose content [J]. Food Science and Biotechnology, 2007, 16(5): 832-835.
[32] IDRIS A, AHMED I. Viscosity behavior of microwave-heated and conventionally heated poly(ether sulfone)/dimethylformamide/ lithium bromide polymer solutions [J]. Journal of Applied Polymer Science, 2008, 108(1): 302-307.
[33] LOBERT M, THIJS H M L, ERDMENGER T, ECKARDT R, ULBRICHT C, HOOGENBOOM R, SCHUBERT U S. Synthesis, microwave-assisted polymerization, and polymer properties of fluorinated 2-phenyl-2-oxazolines: A systematic study [J]. Chemistry—A European Journal, 2008, 14(33): 10396-10407.
[34] WAN Jun-jie. Syntheses and electrochemical properties of poly(amino-quinone)s [D]. Changsha: Central South University, 2004. (in Chinese)
[35] MURALIDHARAN S, RAVICHANDRAN S, PITCHUMANI S, PHANI K L N. Poly (amino-quinone)s: A new class of polymers for anticorrosive applications [J]. Journal of Materials Science Letters, 2000, 19(14): 1299-1301.
[36] WATANABE S, HAYAMA K, PARK K H, KAKIMOTO M, IMAI Y. New microwave-assisted rapid synthesis of polyamides from nylon salts [J]. Die Makromolekulare Chemie, Rapid Communications, 1993, 14(8): 481-484.
[37] MALLAKPOUR S E, HAJIPOUR A R, FAGHIHI K. Microwave-assisted synthesis of optically active poly(amide-imide)s with benzophenone and L-alanine linkages [J]. European Polymer Journal, 2001, 37(1): 119-124.
[38] MALLAKPOUR S, KOWSARI E. Synthesis and properties of organosoluble and optically active poly(amide-imide)s based on epiclon and (s)-(+)-valine under microwave irradiation [J]. Iranian Polymer Journal, 2005, 14(1): 81-90.
[39] MALLAKPOUR S E, HAJIPOUR A R, KHOEE S. Microwave- assisted polycondensation of 4, 4′-(hexafluoroisopropylidene)-N, N′-bis (phthaloyl-L-leucine) diacid chloride with aromatic diols [J]. Journal of Applied Polymer Science, 2000, 77(13): 3003-3009.
[40] GORETZKI C, KRLEJ A, STEFFENS C, RITTER H. Green polymer chemistry: Microwave-assisted single-step synthesis of various (meth) acrylamides and poly (meth) acrylamides directly from (meth) acrylic acid and amines [J]. Macromolecular Rapid Communications, 2004, 25(3): 513-516.
[41] LI Q T, YANG X J, CHEN W Q, YI C F, XU Z S. Preparation of poly(amic acid) and polyimide via microwave-assisted polycondensation of aromatic dianhydrides and diamines [J]. Macromolecular Symposia, 2008, 261: 148-156.
[42] LU J, ZHU X, JI S, ZHU J, CHEN Z. Microwave radiation copolymerization in solid state of maleic anhydride and allylthiourea [J]. Journal of Applied Polymer Science, 1998, 68(10): 1563-1566.
[43] BOGDAL D, PENCZEK P, PIELICHOWSKI J, PROCIAK A. Microwave assisted synthesis, crosslinking, and processing of polymeric materials [J]. Advances in Polymer Science, 2003, 163: 193-264.
Foundation item: Project(50804055) supported by the National Natural Science Foundation of China
Received date: 2009-04-21; Accepted date: 2009-07-15
Corresponding author: LI Hai-pu, PhD, Associate professor; Tel: +86-731-88830603; E-mail: lihaipu@mail.csu.edu.cn
(Edited by YANG You-ping)