Copper solvent extraction from alkaline cyanide solution withguanidine extractant LIX 7950
来源期刊:中国有色金属学报(英文版)2010年第6期
论文作者:F. XIE D. B. DREISINGER
文章页码:1136 - 1140
Key words:copper; cyanide; solvent extraction; guanidine
Abstract: The use of the guanidine extractant LIX 7950 extracting copper and cyanide from alkaline cyanide solution was investigated. The extraction of copper and cyanide under different initial copper and extractant concentrations was examined and the stoichiometric extraction constant of Cu(CN)32- with LIX 7950 was calculated. Both the distribution coefficient and the stoichiometric extraction constant of Cu(CN)32- with LIX 7950 decrease when the temperature is varied from 25 ℃ to 45 ℃, indicating the extraction process is exothermic. The calculated enthalpy change of the reaction (ΔHΘ) is about -190 kJ/mol. The copper extraction isotherms under different molar ratios of cyanide to copper are established. The preferential extraction of Cu(CN)32- over Cu(CN)43- and CN- has been confirmed and a high cyanide-to-copper molar ratio tends to suppress copper loading. The loaded copper and cyanide can be stripped efficiently by the moderately strong NaOH solutions (0.5-1.0 mol/L) and the presence of NaCN in the stripping solution facilitates copper stripping.

F. XIE, D. B. Dreisinger
Department of Materials Engineering, University of British Columbia, Vancouver, BC, Canada V6T 1N6
Received 4 June 2009; accepted 3 September 2009
Abstract: The use of the guanidine extractant LIX 7950 extracting copper and cyanide from alkaline cyanide solution was investigated. The extraction of copper and cyanide under different initial copper and extractant concentrations was examined and the stoichiometric extraction constant of Cu(CN)32- with LIX 7950 was calculated. Both the distribution coefficient and the stoichiometric extraction constant of Cu(CN)32- with LIX 7950 decrease when the temperature is varied from 25 ℃ to 45 ℃, indicating the extraction process is exothermic. The calculated enthalpy change of the reaction (ΔHΘ) is about -190 kJ/mol. The copper extraction isotherms under different molar ratios of cyanide to copper are established. The preferential extraction of Cu(CN)32- over Cu(CN)43- and CN- has been confirmed and a high cyanide-to-copper molar ratio tends to suppress copper loading. The loaded copper and cyanide can be stripped efficiently by the moderately strong NaOH solutions (0.5-1.0 mol/L) and the presence of NaCN in the stripping solution facilitates copper stripping.
Key words: copper; cyanide; solvent extraction; guanidine
1 Introduction
The cyanidation process has been successfully practiced in gold mining industry for more than 100 years[1]. Environmental constraints controlling the discharge of cyanide from gold plants are being tightened by the local governments worldwide after several cyanide spill disasters happened[2]. On the other hand, the common occurrence of copper minerals in gold ores is also a matter of concern to the cyanidation process since most copper minerals are easily and readily soluble in the cyanide solution used in gold leaching, which usually results in high cyanide consumption[3-5]. Detoxifying cyanide by traditional destruction technologies to levels that meet stringent environmental regulations significantly increases the operational cost and may result in a significant economical penalty in loss of valuable copper in cyanide effluent[6]. Acidification, volatilization, and recovery (AVR) and some modifications thereof have been developed and practiced in some gold operations, but the high consumption and cost of reagents have significantly limited the application [7]. The indirect recovery of metals and cyanide with pre-concentration by activated carbon and resins has been proposed; however, the low adsorption capability of carbon and the high cost of resin processes have severely hampered their wide application[8-9].
An alternative method for metal and cyanide recovery is solvent extraction. Quaternary amines and their modified mixtures have been used to extract metal cyanides from high alkaline solutions[10-11]. The extraction and stripping of metal cyanide complexes is believed to occur via an ion-exchange mechanism:
(Q+X-)org+(HP)org+OH-=(Q+P-)org+X-+H2O (1)
where Q+ is the quaternary ammonium cation, HP is the protonated form of the nonylphenol, and X- is the extracted anion[12-13]. In recent years, the recovery of copper and cyanide from waste cyanide solution by guanidine extractants was also suggested[14-15]. These investigations proved that copper can be effectively extracted from alkaline cyanide solutions by the proposed extraction systems and the cyanide levels and the presence of other anions may potentially affect the extraction of copper and cyanide. In this work, continuous laboratory tests on the use of the guanidine extractant LIX 7950 for copper and cyanide recovery from alkaline cyanide solution have been conducted and the distribution constants of copper and the copper extraction isotherms under different experimental conditions were examined. The potential application of the extractant for the recovery of copper and cyanide from waste cyanide solution was discussed.
2 Experimental
2.1 Reagents
LIX 7950 is a trialkylguanidine extractant manufactured by Cognis. The extractant was used as supplied without any further purification. n-dodecane was used as the diluent and 1-dodecanol as the modifier. Synthetic copper cyanide solutions were made up from CuCN and NaCN. All chemicals are of reagent grade.
2.2 Procedures
The extraction and stripping tests were carried out in a sealed beaker and mixing was provided by a mechanical agitator with glass impellers. The temperature was controlled through a thermostatic water-bath. Equilibrium pH was adjusted by direct addition of concentrated H2SO4 solution (10%, volume fraction) or NaOH solution (2 mol/L). Initial investigations showed that the equilibrium between two phases could be established rapidly. In the experiments, contact time of 10 min was arbitrarily chosen for equilibrium establishment. When equilibrium was reached, phase separation was conducted in a separatory funnel. Samples of the aqueous solution were filtered to remove any entrained organic before analysis. The organic samples for stripping were filtered with Whatman 1 PS Phase Separation paper. The copper content in the aqueous sample was analyzed by atomic absorption spectrophotometry (AAS). Total cyanide content in the aqueous solution was determined by a standard distillation method. The copper and cyanide content in the organic phase was calculated by mass balance.
3 Results and discussion
3.1 Effect of copper and extractant concentrations
The extraction of copper and cyanide with LIX 7950 under different copper and extractant concentrations are carried out with relatively dilute copper cyanide solutions ([Cu] = 9.45×10-4 mol/L and 9.45×10-3 mol/L which were equivalent to 60 mg/L and 600 mg/L of copper, respectively, n(CN)/n(Cu)=3, initial pH 10.00±0.05). The organic phase was composed of various amounts of LIX 7950 and 50 g/L 1-dodecanol in n-dodecane. Extraction tests were conducted at an aqueous to organic phase ratio of unity (A/O=1) and 20 ℃. The solution pH was uncontrolled during extraction and the equilibrium pH (pHeq) was measured. It is found that the equilibrium pH for the tests varies in the range of 10-11. According to the copper speciation, Cu(CN)32- is the dominant anion in the aqueous solution which accounts for more than 98% of total copper and cyanide (it may vary in a slight extent depending on the formation constants chosen for the copper cyanide complex species) [15]. The analysis of the extraction of copper and cyanide indicates that the molar ratios of the loaded copper to cyanide are all close to 3 and so are the molar ratio of CN to Cu in the raffinate. It is thus assumed that the overall extraction of copper and cyanide under the experimental conditions is equivalent to the following equilibrium:
2RGorg+2H2O+Cu(CN)32-=2(RGH)?Cu(CN)3,org+2OH-
(2)
Define the stoichiometric extraction constant, Kex-Cu, as
![]() (3)
(3)
where [2(RGH)?Cu(CN)3, org] is the molar concentration of the compound of the extractant and Cu(CN)32- (which is equal to the molar concentration of loaded copper). [RGorg] is the molar concentration of the free extractant in the organic phase. If ideal behaviors for all the related species in both organic and aqueous phases are hypothesized and supposed there is no aggregation of the extractant, [RGorg] can be calculated from the mass balance equation:
[RGorg]=[RGorg]T–2[2(RGH)?Cu(CN)3, org] (4)
where [RGorg]T is the concentration of the total extractant (the initial concentration of the extractant is equivalent to 1.33×10-2 mol/L). The distribution coefficient of copper, DCu, can be expressed as follows:
![]() (5)
(5)
where [Cuorg] and [Cuaq] are the molar concentrations of Cu(CN)32- in the organic phase and in aqueous phase, respectively. Combining Eqs.(3)-(5) leads to the following expression:
lg(DCu[OH-]2)=2lg[RGorg]+lgKex-Cu (6)
Hence, under the ideal conditions, if lg (DCu[OH-]2) is plotted against lg [RGorg], a straight line should be obtained with a slope of 2 and an intercept equals to lg Kex-Cu. The experimental data are presented in Fig.1. A linear relationship with a slope close to 2 is obtained between lg (DCu[OH-]2) and lg [RGorg] when the initial concentration of copper varies from 60 mg/L to 600 mg/L, indicating the effectiveness of Eq.(6). According to the intercept, the logarithm stoichimetric extraction constant of copper with the extractant (lg Kex-Cu) under the experimental conditions is -1.17 which is close to the corresponding average value calculated numerically (-1.34±0.08).
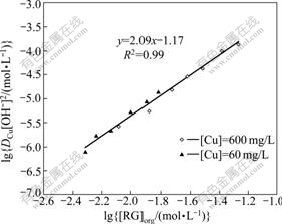
Fig.1 lgDCu[OH-]2 vs lg[RG]org under different copper and extractant concentrations (Organic solvent: LIX 7950 and 50 g/L 1-dodecanol in n-dodecane; aqueous solution: n(CN)/ n(Cu)=3; A/O=1)
3.2 Effect of temperature
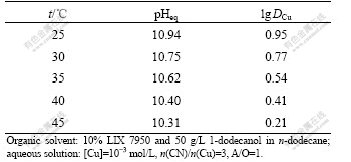
The temperature effects on the extraction of copper and cyanide with LIX 7950 were examined. The relatively dilute copper cyanide solution ([Cu]=9.45× 10-4 mol/L, n(CN)/n(Cu)=3, initial pH 10.00±0.05) and the organic solvent composed of 10% (volume fraction) LIX 7950 and 50 g/L 1-dodecanol in n-dodecane were used. The temperature was varied from 25 ℃ to 45 ℃. The equilibrium pH was measured when the equilibrium was established. The loaded organic solvent was stripped with 1 mol/L NaOH solution for three times. The stripped solvent was re-contacted with the original copper cyanide solution. It was found that the solvents from different temperature tests exhibited the similar extraction capability for copper and cyanide after stripping, indicating that the functionality of the extractant solvents did not change. The extraction results are summarized in Table 1. It can be seen from Table 1 that the equilibrium pH decreases with the increase of temperature. The distribution coefficients for copper and cyanide also decrease when temperature is increased. Similar results were noticed during the extraction of Au(CN)2- by primary amines[16-17]. However, no information about the temperature effect on extraction of metal cyanide complexes by guanidine extractants has ever been reported. The temperature effect can be explained qualitatively in terms of the degree of anions hydration. According to the ion-exchange mechanism (reaction (2)), hydroxyl ions will be released into the aqueous phase when copper cyanide complexes are extracted into the organic phase (i.e., for the extraction of one molecule of Cu(CN)32-, two molecules of OH- will be liberated to the aqueous). Since OH- ion has a relatively large hydration number and a large hydration free energy, it is highly hydrated in water. A typical example is the dissolution of NaOH which is a strong exothermic process due to the large entropy involved in the hydration of OH- ions. There is a possibility that the protonation of the extractant is depressed at an elevated temperature. The solvation energy involving the hydration of copper cyanide complexes are much less compared with that of OH- since they are large anions and less hydrated in water[18-19]. As a result, the overall extraction of copper and cyanide with the extractant is an exothermic process and a high temperature depresses the copper extraction with LIX 7950.
Table 1 Effect of temperature on copper extraction with LIX 7950
The van’t Hoff equation in terms of the extraction constant can be written as
![]() (7)
(7)
where ΔHΘ is the enthalpy change for the extraction reaction (2) and C is the integration constant which is equal to ΔSΘ/(2.303R) under ideal conditions. The values of lgKex-Cu at different temperatures were calculated based on Eq.(3). The plot of lgKex-Cu vs 103/T is presented in Fig.2 and a linear relationship is obtained. It can be seen from Fig.2 that the copper extraction constant (Kex-Cu) decreases when temperature is increased, indicating the overall extraction process is exothermic. The enthalpy change of the reaction was evaluated from the slope which gave a value of -190 kJ/mol (ΔHΘ= -2.303R×103×Slope). ΔSΘ was calculated to be about -0.68 kJ/(K? mol). The negative value of ΔHΘ suggests the formation of the compound of 2(RGH)?Cu(CN)3 is probably a more ordered structure than that of the protonized extractant (RGH+OH-). The extraction of copper cyanides decreases appreciably with an increase of temperature in great extent because of the negative entropy term. Similar results have been reported for the extraction of gold from cyanide solution by tridecylamine (primary amine) and by the solvation extraction system[17, 20]. However, positive values for ΔSΘ were obtained in some primary amine extraction systems[16-17], indicating the effect of temperature on solvent extraction of metal cyanides highly depends on the extraction system adopted, and possibly the properties of the aqueous solution.
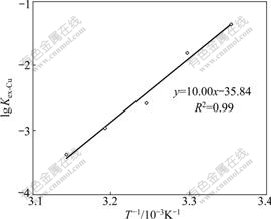
Fig.2 Plot of lgKex-Cu vs 1000/T[15] (10% LIX 7950 in dodecane and 50 g/L 1-dodecanol in n-dodecane; aqueous solution: [Cu]=10-3 mol/L; n(CN)/n(Cu)=3; A/O=1)
3.3 Effect of CN/Cu molar ratios
The extraction isotherms of copper with the extractant under different molar ratios of cyanide to copper and cyanide contents were examined and the results are shown in Fig.3. The extraction tests were carried out at 20 ℃ and the equilibrium pH was controlled at 10±0.05. Fig.3 shows that a high molar ratio of cyanide to copper tends to decrease the copper extraction. The mass balance of copper and total cyanide indicates that the molar ratio of loaded cyanide to loaded copper in organic phase are all close to 3 even when the initial molar ratio of CN to Cu is as high as 10. The analysis of the stripping solutions of the loaded organic samples further confirms this result. The speciation calculation of copper and cyanide indicates that under the experimental conditions, copper mainly occurs as Cu(CN)32- and Cu(CN)43- and cyanide as free cyanide (CN-) and complexed cyanide in the aqueous phase[12, 15, 21]. It can be deduced that the extractant preferentially extracts Cu(CN)32- over Cu(CN)43- and CN-. The phenomenon of preferential extraction and the selectivity order have been explained in detail in the previous work[15, 18, 22]. Basically, the charge density effect and geometrical factors may play the main roles. Cu(CN)32- ion will be preferentially extracted over Cu(CN)43- since the former has a lower charge. Cu(CN)43- has a tetrahedral shape compared with Cu(CN)32- and Cu(CN)2- which are triangularly planar and linear, respectively, and the extraction of Cu(CN)43- would require three extractant molecules per copper while Cu(CN)32- would only require two[12, 23]. As a result, the former is poorly extracted. Since a higher molar ratio of CN to Cu favors the formation of Cu(CN)43-, high cyanide levels depress copper loading. The similar effect of cyanide levels on silver solvent extraction has also been noticed due to the formation of Ag(CN)32- and Ag(CN)43- ions at elevated cyanide levels[20]. Though CN- ion is single charged, its relatively small size leads to the poor extraction with the extractant. As a result, most of the free cyanide will remain in the raffinate solution which allows for the potential recycling of the barren solution to the gold cyanidation process.
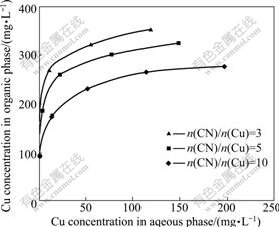
Fig.3 Copper extraction isotherms under different molar ratio of cyanide to copper (10% LIX 7950 in dodecane and 50 g/L 1-dodecanol in n-dodecane; pH=10.50±0.05; 20 ℃)
3.4 Stripping
Varied NaOH and NaCN solutions have been tested for stripping of the loaded copper and cyanide and the results are summarized in Table 2. It can be seen from Table 2 that the loaded copper and cyanide can be effectively stripped by moderately strong NaOH solutions (0.5-1.0 mol/L). At phase ratio of unity, about 93% of copper and cyanide can be stripped off by 1 mol/L NaOH solution. When a concentrated NaOH solution is used, a third phase forms, resulting in a decrease of copper stripping efficiency. The presence of small amount of NaCN in the stripping solution favors the stripping of copper and cyanide. This is probably due to the formation of the less extractable Cu(CN)43- when extra cyanide is present.
Table 2 Stripping of loaded copper and cyanide by NaOH and NaCN solutions (10% LIX 7950 in dodecane and 50 g/L 1-dodecanol in n-dodecane; initial loaded Cu 3.62×10-3 mol/L; total CN 1.10×10-2 mol/L; O/A=1; 20 ℃)

4 Conclusions
1) The extraction of copper and cyanide with the guanidine extractant LIX 7950 under different experiment conditions was examined. The stoichiometric extraction constant of Cu(CN)32- with LIX 7950 was calculated based on the copper extraction under different initial copper and extractant concentrations.
2) Temperature has a significant effect on copper extraction. Both the distribution coefficient and the stoichiometric extraction constant of Cu(CN)32- with LIX 7950 decrease when the temperature is increased from 25 ℃ to 45 ℃ The extraction process is exothermic with the enthalpy change of the reaction (ΔHΘ) of -190 kJ/mol.
3) The preferential extraction of Cu(CN)32- over Cu(CN)43- and CN- has been confirmed and a high cyanide-to-copper molar ratio tends to suppress copper loading. The loaded copper and cyanide can be stripped efficiently by the moderately strong NaOH solutions and the presence of NaCN in the stripping solution facilitates copper stripping.
4) This extractant can be potentially applied to recover copper and cyanide from cyanide effluents or dam return water. A solvent extraction circuit can be incorporated as a pre-concentration step to concentrate copper cyanide into a small volume of strip solution for further recovery of copper products and cyanide by electrowinning, acidification, volatilization and reneutralization, or similar processes. The barren cyanide solution from the SX circuit can be recycled to the cyanidation process.
Acknowledgement
The authors thank Dr. Berend Wassink for his generous help on the project. The project was financially supported by National Science and Engineering Research Council of Canada (NSERC). Cognis is thanked for providing laboratory samples of reagents.
References
[1] Marsden J, House I. The chemistry of gold extraction [M]. London, UK: Ellis Horwood Ltd., 1992: 264-230.
[2] DeVries F. Brief overview of the baia mare dam breach [C]// Cyanide: Social, Industrial and Economic Aspects. New Orleans, US: TMS, 2001: 11-14.
[3] Fleming C A. Cyanide recovery [C]// Advances in Gold Ore Processing. Elsevier, 2005: 703-727.
[4] Jay W H. Copper cyanide recovery system [C]// Cyanide: Social, Industrial and Economic Aspects. New Orleans, US: TMS, 2001: 317-340.
[5] Sceresini B. Gold-copper ores [C]// Advances in Gold Ore Processing. Elsevier, 2005: 789-824.
[6] Botz M M, Mudder T I, Akci A U. Cyanide treatment: Physical, chemical and biological process [C]// Advances in Gold Ore Processing. Elsevier, 2005: 672-702.
[7] Barter J, Lane G, Mitchell D, Kelson R, Dunne R, Trang C, Dreisinger D. Cyanide management by SART [C]//Cyanide: Social, Industrial and Economic Aspects. New Orleans, US: TMS, 2001: 549-562.
[8] Adams M D. Removal of cyanide from solution using activated carbon [J]. Minerals Engineering, 1994, 7(9): 1165-1177.
[9] Le?o V A, Ciminelli V S T, Costa R S. Cyanide recycling using strong-base ion exchange resins [J]. JOM, 1998, 50(10): 66-69.
[10] Moore F L. Solvent extraction of cadmium from alkaline cyanide solutions with quaternary amines [J]. Environmental Letters, 1975, 10(1): 37-46.
[11] Moore F L, Groenier W S. Removal and recovery of cyanide and zinc from electroplating wastes by solvent extraction [J]. Plating and Surface Finishing 1976, 63(8): 26-29.
[12] XIE F, DREISINGER D. Recovery of copper and cyanide from waste cyanide solution by LIX 7820 [J]. Solvent Extraction and Ion Exchange, 2009, 27(4): 459-473.
[13] XIE F, DREISINGER D. Study on solvent extraction of copper and cyanide from water cyanide solution [J]. Journal of Hazardous Materials, 2009, 169: 333-338.
[14] Kordosky G A, Sierokoski J M, Virnig M J, Mattison P L. Gold solvent extraction from typical cyanide leach solutions [J]. Hydrometallurgy, 1992, 30(1/3): 291-305.
[15] Xie F, Dreisinger D. Recovery of copper and cyanide from waste cyanide solution by LIX 7950 [J]. Minerals Engineering, 2009, 22: 190-195.
[16] Caravaca C, Alguacil F J, Sastre A, Martinez M. Extraction of gold(I) cyanide by the primary amine tridecylamine [J]. Hydrometallurgy, 1996, 40: 89-97.
[17] Caravaca C, Alguacil F J , Sastre A, The use of primary amine in gold(I) extraction from cyanide solution [J]. Hydrometallurgy, 1996, 40: 263-275.
[18] Riveros P A. Studies on the solvent extraction of gold from cyanide media [J]. Hydrometallurgy, 1990, 24: 135-156.
[19] Marcus Y. Ion properties [M]. New York: Marcel Dekker Inc., 1985.
[20] Miller J D, Wan R Y, Sibrell P L. Selective salvation of gold from alkaline cyanide solution by alkyl phosphorous esters [J]. Separation Science and Technology, 1987, 22(2/3): 487-502.
[21] Flynn C M, Sandra L M, Cyanide chemistry—Precious metals processing and waste treatment [M]. Montana, US: Bureau of Mines, 1995.
[22] Mooiman M B, Miller J D. The chemistry of gold solvent extraction from cyanide solution using modified amines [J]. Hydrometallurgy, 1986, 16: 245-260.
[23] Sharpe A G. The chemistry of cyano complexes of the transition metals [M]. London, UK: Academic Press, 1976
Corresponding author: F. XIE; Tel/Fax: 1-604-822-3619; E-mail: xiefeng@interchange.ubc.ca
DOI: 10.1016/S1003-6326(09)60268-5
(Edited by LI Xiang-qun)

