A new regeneration process for spent nickel/metal hydride batteries
LI Li(李 丽)1, WU Feng(吴 锋)1,
CHEN Ren-jie(陈人杰)1, GAO Xue-ping(高学平)2, SHAN Zhong-qiang(单忠强)3
(1. School of Chemical Engineering and Environmental, Beijing Institute of Technology, Beijing 100081, China;
2. Institute of New Energy Material Chemistry, Nankai University, Tianjin 300071, China;
3. College of Chemical Engineering, Tianjin University, Tianjin 300072, China)
Abstract: Ultrasonic method was used to recycle nickel/metal hydride(MH-Ni) batteries under undestroyed state. The effects of ultrasonic on electrode material performance of MH-Ni batteries were investigated by using SEM, EDAX and XRD. The results indicate that with the ultrasonic time increasing, there are obvious dispersing phenomena in the positive and negative electrodes. This can make the inertia oxidation layer break off from the negative electrode, and the fresh surface comes out. These changes can increase the reaction centers of the active materials, as well as improve the catalysis capability and discharge ability. But if the ultrasonic time is too long, it can make the active materials reunite and accelerate its pulverization, and lead to its degradation. The improvement of electrochemical performance for MH-Ni batteries is obvious by ultrasonic for 6h continuously.
Key words: MH-Ni batteries; electrode materials; regeneration; ultrasonic method CLC number: TM912
Document code: A
1 INTRODUCTION
Since 1990s, the nickel/metal hydride(MH-Ni) secondary batteries have been extensively applied in various consumer and portable electronic fields, especially in battery-powered electric vehicles(EV) or hybrid electric vehicles(HEV), because of their higher energy density, higher rate capacity, longer cycle life and more friendly to environment than the conventional nickel/cadmium battery. The volume energy density for MH-Ni batteries has doubled from 180W·h·L-1 in 1990 to 360W·h·L-1 in 1997, a value comparable to that for lithium-ion battery[1-3].
Therefore the consumption of MH-Ni batteries is very large and might do great harm to our environment. The rechargeable batteries represent 8% of the European portable battery market. Among the rechargeable batteries, nickel-metal-hydride(MH-Ni) batteries represent 35% of the European market[4-6]. At present, the regeneration of MH-Ni battery is also receiving increasing attention because spent batteries cannot be disposed safely unless metallic materials are properly removed from them due to their explosive nature. As a result of new environmental regulations in different countries around the world, some processes were developed to recycle batteries. To identify the chemical composition is important to promote the recycling of batteries. Unfortunately there is no relationship between the shape and size and the composition[7, 8].
Hydrometallurgical and pyrometallurgical processes can be used to recycle metals present in the batteries. These recycling processes are currently being studied in different parts of the world. Recycling processes for MH-Ni batteries have begun as a function of the economic value associated with nickel, cobalt and rare earths recovery. In Japan, hydrometallurgical processes have been used to recover metals[9]. Mechanical processing to recover nickel has also been evaluated[10, 11]. However, the existing regeneration methods are all on the premise that the battery is disassembled. In this study, a brand-new regeneration process which involves undestroyed state has been first used to recycle MH-Ni batteries.
2 EXPERIMENTAL
2.1 Preparation of electrodes and batteries
The hydrogen storage alloy MlNi3.6Co0.7-Mn0.4Al0.3(where Ml denotes La-rich mischmetal, La 64.6%; Ce 5.9%; Pr 26.6%; Nd 2.2%) was prepared by induction melting from a stoichiometric mixture of Ml, Ni, Mn, Al and Co, followed by a process of annealing at 1000℃. Then, the alloy ingot was mechanically pulverized into 〈74μm. The alloy powder was immersed in a 6mol/L KOH solution at 70℃ for 18h. The negative electrode of Ni/MH battery was formed by mixing the alloy powder with a small amount of nickel and 3%(mass fraction) polytetrafluoroethylene(PTFE), which was pressed into nickel foam substrate. Positive electrode was prepared by filling a nickel foam substrate with Ni(OH)2 mixed with CoO and PTFE. A non-woven polypropylene separator was inserted between the positive and negative electrodes, and then spirally rolled into D-type cylindrical battery cans. A 7mol/L KOH/1mol/L LiOH electrolyte was injected and then the batteries were sealed.
2.2 Charge-discharge cycling and electrochemical performance of MH-Ni batteries
The tests of the charge-discharge cycling were carried out with the scheme that consisted of a charge with 1C for 1.5h, a pause for 10min, and a discharge with 1C to 1.0V after charge-discharge activation at low rate (0.1C). The cycle life of the battery was defined by the cycle number when the discharge capacity was decreased to 80% of the original capacity, which was considered failure and ready to regeneration test by ultrasonic method in undestroyed state. The ultrasonic method was performed at 50℃ and the medium was water. After regeneration, the effects of ultrasonic on electrode material performance of MH-Ni batteries were investigated by using SEM and XRD methods.
Scanning electron microscopy(SEM) and energy dispersive X-ray analysis(EDAX) were carried out using a JSM model 6400 scanning electron microscope equipped with a Noran I-2 EDX unit. The tests were performed to examine the original changes in surface morphology of positive and negative electrodes.
The X-ray diffraction(XRD) patterns of electrodes were obtained on a Rigaku D/max-ⅢA diffractmeter using CuKα radiation and graphite monochromatic filter at 40kV and 100mA. The scan rate is 4.000(°)/min.
3 RESULTS AND DISCUSSION
3.1 Effect of ultrasonic on electrodes
Fig.1 shows the surface morphologies of the positive electrodes after the ultrasonic process for 0, 2, 4, 6, 8h respectively. Table 1 represents the EDAX analysis of the electrode surface. It is obvious that with the ultrasonic time increasing, there are evident dispersing phenomena for the active materials of positive electrode which can increase the active center of the reaction, improve the electrocatalysis performance of the electrode, thus leading to the capacity increasing. But the capacity will decrease if the ultrasonic time is too long. The Ni(OH)2 particles will reunite, which will reduce the active contacted surface.
Table 1 Elements distribution on surface of positive electrode(mole fraction, %)

Fig.2 shows the surface morphologies of the negative electrodes after ultrasonic process for 0, 2, 4, 6, 8h respectively. It is obvious that the surface becomes smoother and has evident metal luster with the ultrasonic time increasing. This indicates that ultrasonic can take effect on metal alloy particles of negative electrode penetrating through the battery case. Table 2 shows the elements distribution on the surface of negative electrode(EDAX). It seems that ultrasonic can make the rare earth oxide fall off from the surface of negative electrode and come out fresh active surface again. This improves the electricity catalysis performance of the electrode and capacity of the battery. Meanwhile, the same phenomenon appears at the positive electrode, and the capacity will decrease if the ultrasonic time is too long. The reason is that the metal alloy particles are pulverized because of excess concussion.
Table 2 Elements distribution on surface of negative electrode(mole fraction, %)
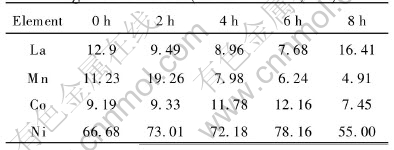
Fig.3 shows the X-ray diffraction patterns for the positive electrodes after different ultrasonic times. The patterns show that the reflections corresponding to γ-NiOOH phase appear before and after ultrasonic test. This phase is formed under conditions of overcharge, high charging and discharging rates, or high electrolyte concentrations. A 44% volume increase is associated with the formation of this phase, so that it causes the swelling or volume expansion of positive electrode[12]. However, compared with the original phase, the intensity of γ-NiOOH phase (003) and (006)
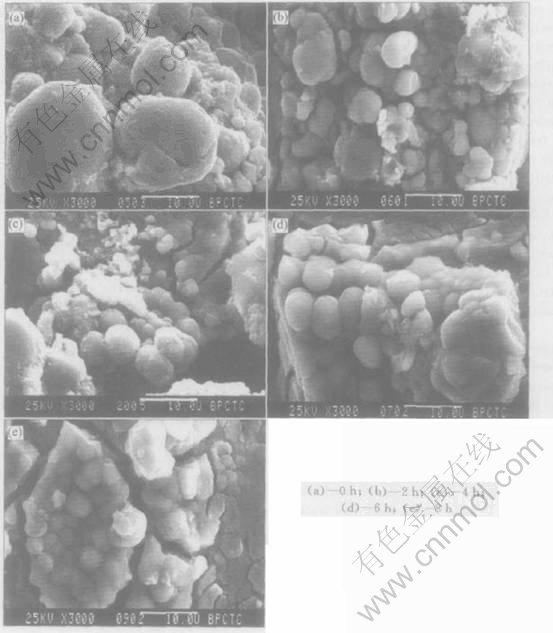
Fig.1 SEM morphologies of positive electrode after ultrasonic processing for different times
decreases after ultrasonic for 2, 4 and 6h respectively, and increases after 8h. This is consistent with the SEM results above. It is possible that the ultrasonic can make the alkali metals ion, hydrogen ion and H2O emerge out from the layer of γ-NiOOH, leading to minishing the distance between two layers to original β-NiOOH. As a consequence, the capacity will be resumed at a certain extent.
Fig.4 shows the X-ray diffraction patterns of the negative electrodes after different ultrasonic times. From the X-ray diffraction patterns for the negative electrodes, it is shown that the alloy after ultrasonic still retains the CaCu5 type structure as the main structure. However, it is found that the intensity of reflection corresponding to Al(OH)3 has no distinct change on the surface of negative electrode, while that of the hydroxides of rare earths(La(OH)3) decreases. This is due to that the inertia oxide layers on the surface of negative electrode can be broken off from electrode and the fresh surface comes out by the process of ultrasonic method[13, 14].
3.2 Effect of ultrasonic on electrochemical performance of MH-Ni battery
The discharge capacity and cycle life of MH-Ni batteries as a function of ultrasonic time are shown in Fig.5 and Fig.6. It can be observed that, after the ultrasonic processing for 6h, the electrochemical performance of the battery is improved obviously. The improvement of discharge capacity at 0.5C rate is 5%, 8.25%, 13.30% and -2.90% respectively, while that of 1C rate is 1%, 7.30%, 7.90% and -2.00% respectively. It is thought that the new active section is formed on the surface of both positive and negative electrodes in order to improve the dynamics performance of alloy electrode. Fig.6 shows the continuous cycle life of batteries after ultrasonic processing for 6h. It is indicated that the cycle life of batteries is increased for 103 cycles. The improvements of electrochemical performance will accelerate the development of lowering the cost of secondary batteries.
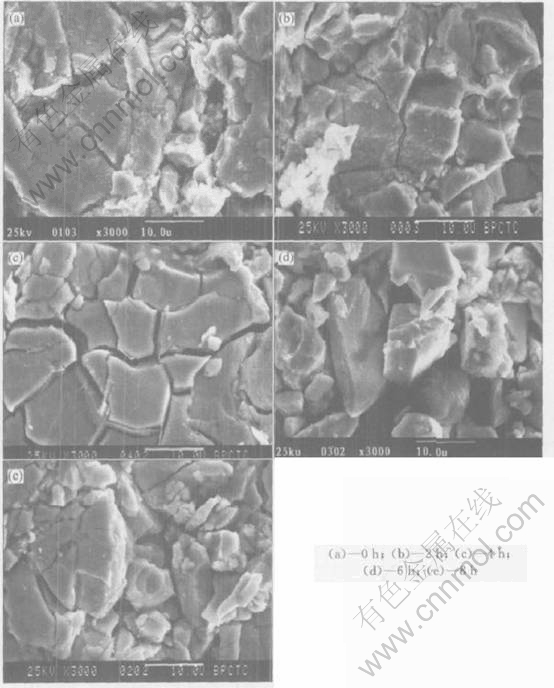
Fig.2 SEM morphologies of negative electrode after ultrasonic processing for different times
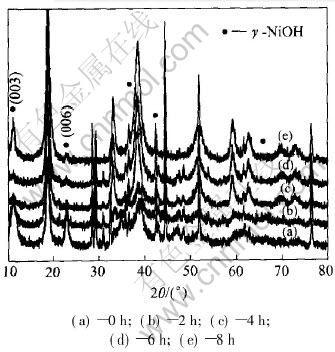
Fig.3 XRD patterns of positive electrodes after different ultrasonic processing times
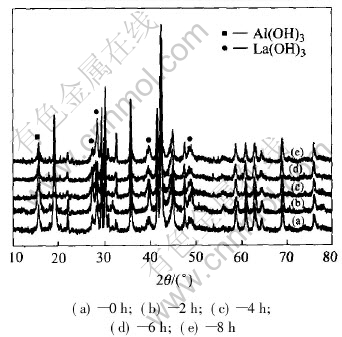
Fig.4 XRD patterns of negative electrodes after different ultrasonic processing times
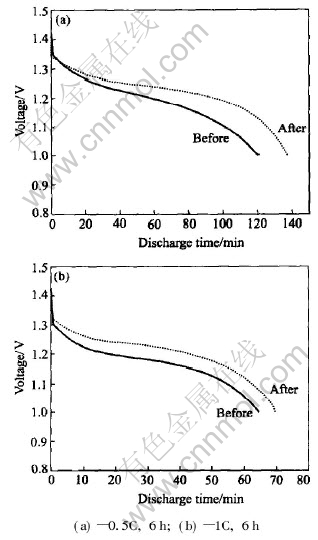
Fig.5 Comparison of electrochemical performance of batteries after different ultrasonic times
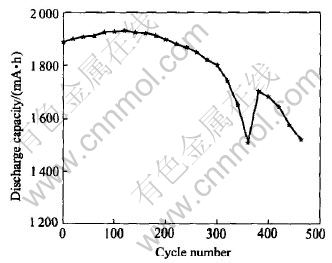
Fig.6 Continuous cycle life of batteries after ultrasonic processing for 6h
4 CONCLUSIONS
An undestroyed regeneration method by using ultrasonic was put forward for recycling spent MH/Ni batteries. Compared with the conventional hydrometallurgical and pyrometallurgical techniques, this new regeneration process has the potential to provide a higher processing efficiency and lower cost of recycling.
To a certain extent, the discharge capacity and cycle life of spent MH-Ni batteries were regenerated by using this new method. With ultrasonic processing time increasing, the inertia oxidation layer can be broken off from the negative electrode, and the fresh surface came out. But if the ultrasonic time is too long, it can make the active materials reunite and accelerate its pulverization, and lead to its degradation again.
REFERENCES
[1]Soria M L, Chacón J, Hernández J C. Metal hydride electrodes and Ni/MH batteries for automotive high power applications [J]. Journal of Power Sources, 2001, 102: 97-104.
[2]Taniguchi A, Fujioka N, Ikoma M, et al. Development of nickel/metal-hydride batteries for EVs and HEVs[J]. Journal of Power Sources, 2001, 100: 117-124.
[3]Nelson R F. Power requirements for batteries in hybrid electric vehicles[J]. Journal of Power Sources, 2000, 91: 2-26.
[4]National Biennial RCRA Hazardous Waste Report: Documents and Data [EB/OL]. http://www.epa.gov/epaoswer/hazwaste/data/biennialreport/index.htm
[5]Bartels J C. How to calculate collection rates for spent batteries [A]. Proceedings of the Fifth International Battery Recycling Congress [C]. Deauville, France, 1999.
[6]Oldershausen N F. VFW-Rebat, a private, profit-oriented battery collection [A]. Proceedings of the Fifth International Battery Recycling Congress [C]. Deauville, France, 1999.
[7]Bernardes A M, Espinosa D C R, Tenório J A S. Recycling of batteries: a review of current processes and technologies[J]. Journal of Power Sources, 2004, 130: 291-298.
[8]Espinosa D C R, Tenório J A S. Fundamental aspects of recycling of nickel-cadmium batteries through vacuum distillation[J]. Journal of Power Sources, 2004, 135: 320-326.
[9]Zhang P, Yokoyama T, Itabashi O, et al. Recovery of metals from spent-metal hydride rechargeable batteries [J]. Journal of Power Sources, 1999, 77: 116-122.
[10]Tenório J A S, Espinosa D C R. Recovery of Ni-based alloys from spent Ni/MH batteries [J]. Journal of Power Sources, 2002, 4707: 1-4.
[11]WANG Rong, YAN Jie, ZHOU Zhen. Regeneration of hydrogen storage alloy in spent nickel-metal hydride batteries [J]. Journal of Alloys and Compounds, 2002, 336: 237-241.
[12]Singh D. Characteristics and effect of γ-NiOOH on cell performance and a method to quantify it in nickel electrodes [J]. J Electrochem Soc, 1998, 145: 116-119.
[13]LI Li, WU Feng, YANG Kai. Degradation behavior of electrochemical performance of sealed-type nickel/metal hydride batteries [J]. Journal of Rare Earths, 2003, 21(3): 341-346.
[14]LI Li, WU Feng, YANG Kai, et al. Electrochemical performance of nickel/metal hydride batteries under unconventional conditions and degradation analysis[J]. Trans Nonferrous Met Soc China, 2004, 14(1): 208-214.
(Edited by YUAN Sai-qian)
Foundation item: Project(2002CB211800) supported by the National Basic Research Program of China; Project(2001CCA05000) supported by the National Key Program for Basic Research of China
Received date: 2004-12-09; Accepted date: 2005-03-11
Correspondence: WU Feng, Professor; Tel: +86-10-68912508; Fax: +86-10-68451429; E-mail: wufeng863@vip.sina.com