Adsorption of avermectins on activated carbon: Equilibrium, kinetics, and UV-shielding
来源期刊:中国有色金属学报(英文版)2009年增刊第3期
论文作者:顾微 孙长娇 刘琪 崔海信
文章页码:845 - 850
Key words:activated carbon; avermectins; adsorption; UV-shielding
Abstract: The adsorption of biopesticide avermectins onto activated carbon from ethanol solution with different initial concentrations at 303.15 K was performed. The obtained equilibrium and kinetic data of the adsorption process were assayed to evaluate the adsorption potential of activated carbon for avermectins. The results show that the activated carbon is effective for the adsorption of avermectins. Moreover, the adsorption of avermectins onto activated carbon agrees with Langmuir isotherm model, while pseudo-second-order kinetics model is better fitable for such adsorption process. In addition, activated carbon can efficiently protect adsorbed avermectins from photodegradation.
基金信息:the National High-Tech Research and Development Program of China

GU Wei(顾 微), SUN Chang-jiao(孙长娇), LIU Qi(刘 琪), CUI Hai-xin(崔海信)
Institute of Environment and Sustainable Development in Agriculture, Chinese Academy of Agricultural Sciences, Beijing 100081, China
Received 10 August 2009; accepted 15 September 2009
Abstract: The adsorption of biopesticide avermectins onto activated carbon from ethanol solution with different initial concentrations at 303.15 K was performed. The obtained equilibrium and kinetic data of the adsorption process were assayed to evaluate the adsorption potential of activated carbon for avermectins. The results show that the activated carbon is effective for the adsorption of avermectins. Moreover, the adsorption of avermectins onto activated carbon agrees with Langmuir isotherm model, while pseudo-second-order kinetics model is better fitable for such adsorption process. In addition, activated carbon can efficiently protect adsorbed avermectins from photodegradation.
Key words: activated carbon; avermectins; adsorption; UV-shielding
1 Introduction
Avermectins (scheme 1), a class of macrocyclic lactones isolated from the soil organism streptomyces avermitilis, show a broad spectrum of agricultural pesticidal activity. Due to advantages of high efficiency, low toxicity and high selectivity, avermectins now are becoming a class of the most popular products in the biological pesticide market. However, avermectins are sensitive to several environmental factors, especially to UV light. The presence of UV light leads to a rapid photo-oxidative degradation of avermectins, thus resulting in a very short half-life of avermectins[1]. In order to improve the pestcidal activity of avermectins, it is preferable to adsorbing the avermectins onto some forms of adsorbent that can protect avermectins from degradation and consequently prevent the loss of pesticidal activity.

Scheme 1 Chemical structure of avermectins (R1= H or CH3 and R2 = CH3 or C2H5)
Activated carbon exhibits a high degree of porosity and an extended internal surface area. Therefore, it has been extensively used for the purpose of purification, e.g., for the purification of water and air[2]. In recent years, however, it has been increasingly used for the prevention of environmental pollution[3-5] and in pharmaceutical areas as well[6]. In both cases, the adsorptive properties of the activated carbons at the solid-liquid interface are of the utmost importance because the physico-chemical knowledge of solid surfaces is interesting, not only for purely scientific reasons but also for their practical implications.
The toxicity of pesticides and their degradation products poses a potential hazard to environment. Previous studies show that the adsorption of pesticides on activated carbon provides an effective approach to remove the residual of pesticide from environment [7-9]. However, absorption characteristics of activated carbon for biopesticide and, more important, the effect of activated carbon on protecting biopesticide from photodegradation have not been studied so far. In this work, activated carbon with a BET surface area greater than 1 500 m2/g was employed as an absorbent to adsorb avermectins from ethanol solution with different initial concentrations at 303.15 K. Equilibrium and kinetic data of the adsorption process were then assayed to provide fundamental information required to evaluate the affinity or capacity of the adsorbent and to understand the adsorption mechanism of avermectins compounds onto the activated carbon. Specifically, the applicability of the isotherm models, that is, Langmuir isotherm and Freundlich isotherm, was analyzed by comparing the correlation coefficients. Pseudo-first-order and pseudo- second-order kinetics models were adopted to examine the mechanism of the adsorption process of avermectins by activated carbon. In addition, the UV-shielding property provided by activated carbon for the protection of adsorbed avermectins from photodegradation was examined.
2 Experimental
Avermectins of 95% purity were obtained from Zhejiang Qianjiang Biochemical Co., Ltd, China. Activated carbon was generously provided by Tsinghua University as a gift. Other chemicals were purchased from Beijing Chemical Factory, China. All chemicals were analytical grade and used as received.
Batch equilibrium studies were carried out by adding 500 mg activated carbon into a series of 250 mL Erlenmeyer flasks with 100 mL avermectins ethanol solution at different concentrations. The flasks were maintained at 303.15 K for 24 h. After centrifuging, the absorbance of equilibrium avermectins in supernatant was determined by UV-Vis spectrophotometer (UNIC 2800, China) at 254 nm and the corresponding concentrations of avermectins were then computed using a standard calibration curve. The amount of adsorbed avermectins at equilibrium, Qe, was calculated by
![]() (1)
(1)
where C0 and Ce are the concentrations of avermectins at initial and equilibrium stages, respectively; V is the volume of the suspension; and m is the mass of activated carbon used.
To study the adsorption kinetics together with the effect of avermectin initial concentration and contact time on the adsorption, 500 mg activated carbon was dispersed in 100 mL ethanol solution with initial avermectins concentrations of 2, 4 and 6 g/L at 303.15 K, respectively. At predetermined intervals of time, suspensions were sampled and centrifuged. The supernatant was then analyzed for the determination of equilibrium concentration of avermectins by measuring the absorbance of avermectins at 245 nm. The amount of adsorption Qt at time t was then calculated according to the following equation:
![]() (2)
(2)
where Ct is the concentration of avermectins at time t.
The UV-shielding property of activated carbon for avermectins was evaluated by measuring the change of avermectins as the uniformly dispersed thin film upon exposure to UV light. Briefly, 5 mL ethanol suspension of avermectins-loaded activated carbon was placed in several uncovered glass Petri dishes and dried in air at room temperature to form thin films. The as-prepared thin films were then placed under a UV light with λ= 365 nm and removed from the UV light after 24 and 72 h UV irradiation, respectively. Avermectins-loaded activated carbon was then recovered by rinsing thin film with 5 mL ethanol, followed by stirring for 30 min. The obtained suspension was centrifuged and the supernatant was collected and analyzed by monitoring the absorbance at 245 nm to determine the concentration of remaining avermectins. For the comparison, the degradation of free avermectins thin film under the same conditions was performed.
3 Results and discussion
3.1 Effect of contact time and initial concentration on avermectins adsorption by activated carbon
The adsorption of avermectins by activated carbon was studied at different initial avermectins concentrations (2, 4, and 6 g/L). Fig.1 shows the plots for the effect of initial concentration and contact time on the adsorption of avermectins by activated carbon at 303.15 K. It shows that the adsorption of avermectins increases with the increase of contact time, and reaches equilibrium at a certain time. The amount of avermectins adsorbed at the equilibrium time reflects the maximum adsorption of the adsorbent under the operating conditions applied. The results reveal that avermectins adsorption is fast at the initial stage of the contact period, and thereafter it becomes slower as approaching to the equilibrium. This phenomenon is due to the fact that a large number of vacant surface sites are available for adsorption during the initial stage, and after a lapse of time, the remaining vacant surface sites are difficult to be occupied due to repulsive forces between the solute molecules on the solid and bulk phases.
As can also be seen from Fig.1, the amount of the adsorbed avermectins at a high initial concentration of 6 g/L achieves adsorption equilibrium in approximately 4 h, while at low initial avermectins concentrations (2 and 4 g/L), the time necessary to reach equilibrium is more than 12 h. Therefore, in this work, the experimental data were measured up to 48 h to ensure that the complete equilibrium was attained. Furthermore, it reveals that the initial concentration plays an important role in the adsorption of avermectins on activated carbon. As observed from Fig.1, an increase in initial avermectin concentration leads to an increase in the adsorption of avermectins on activated carbon. This is likely due to a larger mass transfer driving force caused by the increase of initial concentration, hence resulting in larger adsorption amount of avermectins by activated carbon.
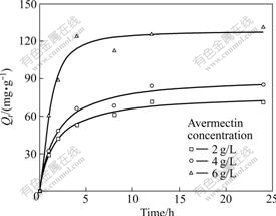
Fig.1 Effect of initial concentration and contact time on avermectins adsorption onto activated carbon at 303.15 K
3.2 Modeling of adsorption isotherms
The adsorption isotherm indicates the adsorption molecules distribution between the liquid phase and the solid phase when the adsorption process reaches an equilibrium state. Therefore, adsorption isotherm is basically important to describe the interaction of solutes with adsorbents, and is critical in optimizing the use of adsorbents. Moreover, parameters of the isotherm model equations are related to the structural properties of the materials, which are crucial to the selection of the solids used as adsorbents.
Adsorption equilibrium data of avermectins on activated carbon were fitted by applying Langmuir and Freundlich isotherm models, respectively. The applicability of the isotherm models to the adsorption study was compared by judging the correlation coefficients.
The Langmuir adsorption model is based on the assumption that the maximum adsorption corresponds to a saturated monolayer of solute molecules on the adsorbent surface, with no lateral interaction between adsorbed molecules[10-11]. The linear expression of the Langmuir model is given by
![]() (3)
(3)
where Qe and Ce represent the amount of adsorbed avermectins per unit mass of adsorbent and avermectins concentration at equilibrium, respectively; Q0 is the maximum amount of avermectins per unit mass of adsorbent to form a complete monolayer on the surface bound at high Ce; and b is a constant related to the affinity of binding sites.
Freundlich model, on the other hand, is an empirical equation based on sorption on a heterogeneous surface. It is assumed that the stronger binding sites are occupied first and then the binding strength decreases with the increase of the degree of site occupation[12]. The well-known logarithmic form of Freundlich isotherm is depicted in the following equation:
![]() (4)
(4)
where KF is the Freundlich constant that is defined as the adsorption or distribution coefficient and represents the quantity of dye adsorbed onto activated carbon at a unit equilibrium concentration. The slope of 1/n ranging from 0 and 1 is a measure of adsorption intensity or surface heterogeneity, which becomes more heterogeneous as its value gets closer to zero. If the plot of lg Qe vs lg Ce gives a straight line, the slope will be 1/n and the intercept will be lg KF.
The correlation coefficients, R2, obtained from two isotherm models applied for the adsorption of avermectins on the activated carbon were obtained from Fig.2. The Langmuir model gives a higher R2 than Freundlich model, suggesting that the adsorption of avermectins on activated carbon can be better represented by Langmuir isotherm.
3.3 Adsorption kinetics
The kinetics of adsorption of avermectins on activated carbon was investigated by two commonly used models, namely, the Lagergren pseudo-first-order model and the pseudo-second-order model.
First, the kinetics of adsorption of avermectins on activated carbon was analyzed by the pseudo-first-order equation as follows[13]:
![]() (5)
(5)
where k1 is the rate constant of the adsorption. If the plot is linear with large correlation coefficient, Lagergren’s equation will be appropriate to avermectins adsorption on activated carbon. However, R2 values obtained from Fig.3(a) are relatively small, which are 0.960, 0.941, and 0.883 at avermectins initial concentrations of 2, 4, and 6 g/L, respectively.
Next, the kinetics of adsorption was examined by the pseudo-second-order equation, which is based on
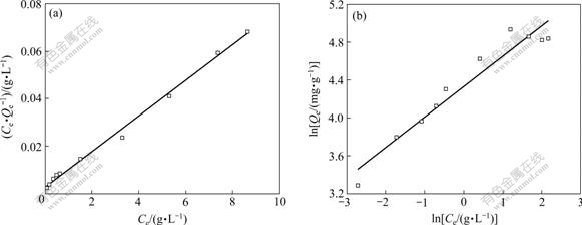
Fig.2 Langmuir isotherm model (a) and Freundlich isotherm model (b) of avermectins adsorption by activated carbon
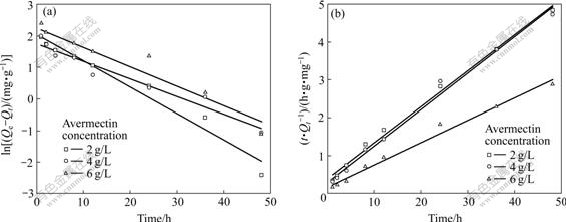
Fig.3 Pseudo-first-order kinetics model (a) and pseudo-second-order kinetics model (b) for avermectins adsorption on activated carbon at 303.15 K
equilibrium adsorption and can be expressed as[14]:
![]() (6)
(6)
where k2 is the rate constant of second-order adsorption. This procedure is more likely to predict the behavior over the whole range of adsorption. The linear plot of t/Qt vs t at 303.15 K is shown in Fig.3(b). R2 as obtained is greater than 0.98 at all initial concentrations of avermectins, indicating the applicability of this model to describe the adsorption process of avermectins onto activated carbon.
3.4 Intraparticle diffusion model
In order to gain insight into the mechanisms and rate controlling steps affecting the kinetics of adsorption, experimental data were fitted to Weber’s intraparticle diffusion model[15], which is expressed as
![]() (7)
(7)
where C is the intercept and kp is the intraparticle diffusion rate constant, which can be evaluated from the slope of the linear plot of Qt vs t1/2. The intercept of the plot reflects the boundary layer effect, that is, the larger the intercept, the greater the contribution of the surface adsorption in the rate-controlling step. If the regression curve of Qt vs t1/2 is linear and passes through the origin, then intraparticle diffusion will be the sole rate-limiting step. However, the linear plot for each concentration that passes through the origin is not the case in this work, as shown in Fig.4, indicating that the intraparticle diffusion is not the only rate controlling step. As a matter of fact, external mass transfer of biopesticide molecules onto activated carbon is also significant in the sorption process, especially in the initial adsorption period. Therefore, intraparticle diffusion plots are then divided into two linear stages (Fig.4). The first sharper region is the instantaneous adsorption or external surface adsorption, and the second region is the gradual adsorption stage where intraparticle diffusion is the rate limiting. Referring to Fig.4, at all initial concentrations, the first stage is completed in the first 4 h and the second stage of intraparticle diffusion control is then attained. Furthermore, different stages of adsorption rates observed indicate that the adsorption rate is initially faster and then slows down. As can also be seen from Fig.4, the linear lines of the second stage do not pass through the origin. This deviation from the origin may be due to the difference in the mass transfer rate in the initial and final stages of adsorption.
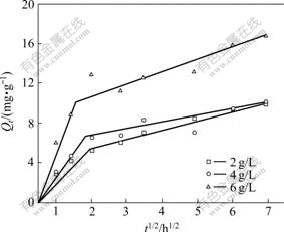
Fig.4 Intraparticle diffusion model for adsorption of avermectins on activated carbon at 303.15 K
3.5 UV shielding property of activated carbon for adsorbed avermectins
The UV shielding property of activated carbon provided for adsorbed avermectins was evaluated by the thin-film method described in Ref.[1]. Fig.5 shows the changes of normalized concentrations of avermectins (C/C0), i.e. the ratio of remaining avermectins concentrations to the initial avermectins concentrations, under the UV irradiation for 0, 24 and 72 h for free and adsorbed avermectins, respectively. It is found that 40% of free avermectins is decomposed after 24 h UV irradiation in this experimental condition. However, for avermectins adsorbed on activated carbon, less than 2% of avermectins is degraded after the same UV irradiation time, indicating that adsorbed avermectins are well protected by activated carbon from UV degradation. Although prolonging UV irradiation time (up to 72 h) leads to the photodegradation for both free and adsorbed avermectins, the higher degradation degree for free avermectins (70%) as compared to that of adsorbed avermectins (25%) further confirms the capability of activated carbon for protecting adsorbed avermectins from photodegradation.
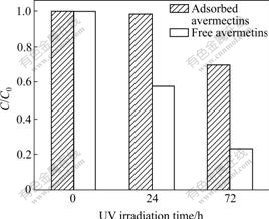
Fig.5 Change in normalized concentration of free and adsorbed avermectins as function of UV irradiation time
4 Conclusions
1) Activated carbon with high BET surface area is able to adsorb biopesticide avermectins from ethanol solution with different initial concentrations at 303.15 K.
2) An increase in the initial concentration of avermectins results in an increase in the adsorption amount of avermectins on activated carbon.
3) The adsorption equilibrium and kinetics of avermectins by activated carbon show that the adsorption equilibrium of avermectins by activated carbon can be best fitted by the Langmuir isotherm model, while the pseudo-second-order kinetic model the best fitted for the adsorption data.
4) Activated carbon can effectively protect adsorbed avermectins from photodegradation.
References
[1] FEELY W F, CROUCH L S, ARISON B H, VANDENHEUVEL W J A, COLWELL L F, WISLOCKI P G. Photodegradation of 4″-(epimethylamino)-4″-deoxyavermectin-B1a thin-films on glass [J]. Journal of Agricultural and Food Chemistry, 1992, 40(4): 691-696.
[2] OTOWA T, NOJIMA Y, MIYAZAK T. Development of KOH activated high surface area carbon and its application to drinking water purification [J]. Carbon, 1997, 35(9): 1315-1319.
[3] FAHIM N F, BARSOUM B N, EID A E, KHALIL M S. Removal of chromium(Ⅲ) from tannery wastewater using activated carbon from sugar industrial waste [J]. Journal of Hazardous Materials, 2006, 136(2): 303-309.
[4] KIM T Y, JIN H J, PARK S S, KIM S J, CHO S Y. Adsorption equilibrium of copper ion and phenol by powdered activated carbon, alginate bead and alginate-activated carbon bead [J]. Journal of Industrial and Engineering Chemistry, 2008, 14(6): 714-719.
[5] VALINUROVA E, KADYROVA A, SHARAFIEVA L, KUDASHEVA F. Use of activated carbon materials for wastewater treatment to remove Ni(Ⅱ), Co(Ⅱ), and Cu(Ⅱ) ions [J]. Russian Journal of Applied Chemistry, 2008, 81(11): 1939-1941.
[6] CASAS R N, RODRIGUEZ A G, BUENO F R, LARA A E, CALAHORRO C V, GUIJOSA A N. Interactions of xanthines with activated carbon (Ⅰ). Kinetics of the adsorption process [J]. Applied Surface Science, 2006, 252(17): 6022-6025.
[7] HAMEED B H, SALMAN J M, AHMAD A L. Adsorption isotherm and kinetic modeling of 2,4-D pesticide on activated carbon derived from date stones [J]. Journal of Hazardous Materials, 2009, 163(1): 121-126.
[8] DANESHVAR N, ABER S, KHANI A, RASOULIFARD M H. Investigation of adsorption kinetics and isotherms of imidacloprid as a pollutant from aqueous solution by adsorption onto industrial granular activated carbon [J]. Journal of Food Agriculture and Environment, 2007, 5(3/4): 425-429.
[9] DOMINGUES V E, PRIOLO G, ALVES A C, CABRAL M E, DELERUE-MATOS C. Adsorption behavior of alpha-cypermethrin on cork and activated carbon [J]. Journal of Environmental Science and Health Part B—Pesticides Food Contaminants and Agricultural Wastes, 2007, 42(6): 649-654.
[10] LANGMUIR I. The constitution and fundamental properties of solids and liquids (Part Ⅰ). Solids [J]. Journal of the American Chemical Society, 1916, 38(11): 2221-2295.
[11] LANGMUIR I. The constitution and fundamental properties of solids and liquids (Part Ⅱ). Liquids [J]. Journal of the American Chemical Society, 1917, 39(9): 1848-1906.
[12] FREUNDLICH H M F. Over the adsorption in solution [J]. J Phys Chem, 1906, 57: 385-470.
[13] LANGERGREN S, SVENSKA B K. Zur theorie der sogenannten adsorption gel?ster stoffe [J]. Veternskapsakad Handlingar, 1898, 24(4): 1-39. (in German)
[14] HO Y S, MCKAY G. The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat [J]. The Canadian Journal of Chemical Engineering, 1998, 76(4): 822-827.
[15] WEBER W J, MORRIS J C. Kinetics of adsorption on carbon from solution [J]. Journal of the Sanitary Engineering, 1963, 89: 31-39.
(Edited by CHEN Wei-ping)
Foundation item: Projects(2006AA10A203; 2007AA021808) supported by the National High-Tech Research and Development Program of China
Corresponding author: CUI Hai-xin; Tel: +86-10-82106013; E-mail: haixin-cui@hotmail.com

