Article ID: 1003-6326(2005)06-1346-05
Anti-oxidation mechanism of SiC-B4C-C composites
LIU Qi-cheng(刘其城)1,2, XU Xie-wen(徐协文)1,2,
HUANG Qi-zhong(黄启忠)2, HUANG Bai-yun(黄伯云)2
(1. Department of Materials Science and Egineering,
Changsha University of Science and Technology, Changsha 410077, China;
2. State Key Laboratory of Powder Metallurgy, Central South University,
Changsha 410083, China)
Abstract: Mixture of green petroleum coke, B4C and SiC together with short carbon fiber were employed as starting materials, the mixture was press-formed without any binder after grinding, dense and homogeneous binderless SiC-B4C-C(carbon/ceramic) composites were then obtained after sintering. Composites thus prepared possess excellent anti-oxidation property, that is, mass loss less than 1% within the temperature range from 900 to 1100℃ for 10h. Anti-oxidation mechanism was also discussed from the viewpoint of thermodynamics, excellent anti-oxidation property of materials thus prepared can be discribed to 1) solid SiO2 formed from SiC, which restrains the filtering of oxygen and simultaneously, its volume expansion brought about by the reaction takes roles both walling up the original pores and making the material more compact; 2) liquid B2O3 from the reaction of B4C not only makes the combination with C, B4C and SiC tighter through forming solid solution, but also effect of reaction SiC(s)+2CO(g)=SiO2(s)+3C(s) is an expansive process, which improves the microstructure of the material.
Key words: SiC-B4C-C composites; anti-oxidation; mechanism CLC
number: TQ050.4 Document code: A
1 INTRODUCTION
C/C composites with excellent properties of carbon fiber and matrix carbon widely used in metallurgy, machinery, electric, nuclear energy and aeronautics and space, are a kind of fast growing and prosperous new material. Recently it has been used as exhausting pipe for rocket and missile owing to its good anti-thermal-shock property, but the obvious shortcoming of it limits its application, that is, the nearly half mass loss of its binder during heat treatment process makes this material have high porosity and low strength, especially low anti-oxidation property[1]. To overcome that disadvantage, protection film such as dipping phosphate or borate is employed, or SiC or B4C (or other carbide) is doped to form a SiC-B4C-C(carbon/ceramic) composite[2-6]. These improvement methods are effective to promoting its high-temperature anti-oxidation property, mechanical strength and thermal conductivity. Adding SiC as protection coating in inert atmosphere at 1450℃ was discussed[6], Chinese scholars[7] also mentioned SiC as composite coating and its protecting ways; others [8] proposed TiC/SiC protection coating layer. While that increases its preparation cost and producing period, and makes it difficult for industrial production.
In this paper, the authors discuss a new process in which green petroleum coke, SiC and B4C powder were mixed, and ground, then formed without binder (which can be called binderless forming), and compact carbon/ceramic were obtained after sintering. This approach, characterized by its simplicity and short processing period, can prepare SiC-B4C-C(carbon/ceramic) composites with both excellent anti-oxidation property and high strength[9-11]. The oxidation mass loss (the indicator of anti-oxidation index), and further the anti-oxidation mechanism with regard to thermo-dynamics were also discussed.
2 EXPERIMENTAL
2.1 Preparation of specimens
The raw materials used are as follows: Jingmen green petroleum coke (prepared with post-poning method, with average diameter 5μm), pre-oxidized PAN carbon fiber (Shanghai Carbon Plant, average length 3-5mm), and SiC and B4C ceramic powder (ground to 1-2μm). Milling SiC and B4C ceramic powder (in various ratios) with the green petroleum powder 24h, adding in carbon fiber, and then model pressed. Heat treatment was carried out in N2 atmosphere at 2000℃ sintering at 1100℃, the carbon/ceramic composites were obtained, Table 1 gives the composition of the specimens, followed by processing flow chart (Fig.1).
Table 1 Composition of specimen(mass fraction, %)

Fig.1 Processing flow chart
2.2 Testing of prepared specimens
Oxidation mass loss was tested at different oxidation temperatures and oxidation times at the same air flow (50mL/min). The oxidation temperatures were arranged at 900, 1000, 1100 and 1200℃ respectively with the specimen dimension of 40mm×10mm×10mm. Phase analysis and testing were carried out by X-ray diffractometry(XRD) and its microstructure was analyzed with scanning electronic microscopy(SEM). Thermo-dynamic mechanism of the anti-oxidation property was discussed on the basis of these tests.
3 EXPERIMENTAL RESULTS
3.1 Anti-oxidation property at different temperatures
Fig.2 reveals that oxidation mass loss increases with the increase of temperature obviously for specimen No.1, while No.2 has a lower loss—less than 1% below 1100℃; No.3 and 4 have greater mass loss at lower temperature but smaller loss at higher temperature, oxidation time for all specimen is 10h. Therefore it can be seen that specimen No.2 possesses a good anti-oxidation property both at lower and higher temperatures.

Fig.2 Oxidation mass losses at different temperatures

Fig.3 Oxidation mass losses at different oxidation times
3.2 Anti-oxidation property at different times
Fig.3 is drawn based on the above experimental results, which indicates the relationship between oxidation mass loss and oxidation time of specimen No.2. Oxidation mass loss of the four specimens varying with the oxidation time can be shown in Fig.3, which is obvious that the oxidation mass loss at lower temperature (900℃) is higher than that at higher temperature (1200℃) at air flow of 50mL/min and respective oxidation time of 5, 10 and 15h.
Figs.4 (a) and (b) illustrate the SEM images of the specimen before and after oxidation (heat treatment 10h), which shows the change of microstructure, but not much difference happens after heat treatment, the surface micro-porosity does not alter. The above discussion indicates that it is the SiC and B4C ceramic additives, not the short oxidation time contributes most to the improvement of the increase of the anti-oxidation property of C/C composites. Products without ceramic additives have an oxidation mass loss [1] as high as 45% and high porosity even at the oxidation time of 2h at 550℃.

Fig.4 SEM images before and after oxidation mass loss
4 DISCUSSION OF OXIDATION MECHANISM
4.1 Oxidation model
It is generally accepted that the multi-phase oxidation reaction takes place on the active surface, that is, the so called oxidation centers. The most common oxidation centers are cavity, dislocation (line defect), the other structural defects and the edge atoms, the adding of ceramic additives makes the oxidation process more intricate. The oxidation process can be simplified in Fig.5[12]: when the right side of C/C composites has already decarbonized, its carbon has been oxidized. The newly formed pore together with the original one becomes the diffusion path (dashed line). In this case the oxidation process of carbon can be thought as: oxygen passes through the surface of the composites, enters the inner part of the material through diffusion path, then reacts with carbon when it reaches the gas-solid interface, and the reacted product-carbon dioxide passes out along the diffusion path. Therefore, through the above brief discussion, the additive candidate should possess the following two characteristics, they are: 1) it has a greater affinity with oxygen than that with carbon atom and 2) the compound(s) formed with O2, CO, or carbon brings about a change of microstructure of the composites, such as an increase of density, a wall-up of pores and a slow-down of the diffusion of reactant(s). Experimental results illustrate that the additives employed possess the above characteristics.

Fig.5 Oxidation model of C/C composites
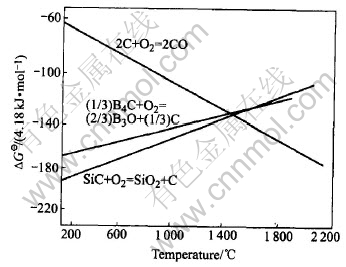
Fig.6 Standard oxidation reaction free energy of related atoms in carbon product reaction system
Fig.6[12] show the relation line between the reaction (between ceramic powder and oxygen) free energy and temperature, which reveals that the affinity between ceramic powders employed and oxygen are all greater than that between carbon and oxygen, thus ceramic powder(s) is preferentially oxidized prior to carbon (powder and fiber) to form an oxidation protection film, which hinders much diffusion path, and that is why it restrains the further oxidation.
4.2 Thermodynamic analysis
XRD pattern in Fig.7 shows the mineral (phase) composition of specimen before oxidation; it contains C, SiC, B4C and B2O3. The formal three compositions are raw materials by all appearances, while B2O3 is the product of reaction between B4C and oxygen, which is the basis of thermodynamic analysis.

Fig.7 XRD pattern of specimen before oxidation

Fig.8 SiO2-SiC-C phase diagram at 1800K and different CO pressures
1) Reaction mechanism of SiC
Fig.8[13] shows the significant role of SiC on anti-oxidation. SiC is thermally unstable at 1800K and p(CO)=1MPa, and forms SiO2 (s) and C(s) as follows:
SiC(s)+2CO(g)=SiO2(s)+3C(s)(1)
with a free energy
ΔG=-616297+11.43TlgT+303.5T-
38.31Tlgp(CO)
When p(CO)=1MPa, and the temperature is lower than 1100℃, all the ΔG values are less than zero, that is, when the temperature is not higher than 1100℃, the above reaction tends to proceed from left to right. Description is[10] : first, SiC(s) reacts with CO(g) to form C(s) and SiO(g):
SiC(s)+CO(g)=SiO(g)+2C(s)(2)
Carbon formed deposes onto the surface of SiC, which increases p(CO) and decreases p(SiO), while gaseous SiO diffuses and reacts with neighboring CO to form SiO2 and C(s) as follows:
SiO(g)+CO(g)=SiO2+C(s)(3)
The above reaction makes CO reduced to carbon, and SiO2 formed enwraps on the surface of C, B4C and SiC to restrain oxidation infiltration and inhibit further oxidization of carbon, simultaneously, the volume expansion of formed SiO2 and C wall up in the original cavity, which increases the density and anti-oxidation ability of the material.
2) Reaction mechanism of B4C
Fig.9[13] is an illustration of thermodynamic stable areas of various phases in B-O-C system. It can be see that B4C remains stable at 1327℃ only on condition that lgp(CO)≤1.285: while in oven, B4C is stable at 1000℃ with partial pressure approximately 0.1MPa and reacts with CO and O2 as follows[14]:
(1/3)B4C(s)+O2(g)=
(2/3)B2O3(l)+(1/3)C(4)
(1/2)B4C(s)+3CO(g)=
B2O3(l)+(7/2)C(s)(5)
(1/2)B4C(s)+2CO(g)=
B2O2(g)+(5/2)C(s)(6)
(1/4)B4C(s)+CO(g)=BO(g)+(5/4)C(s)(7)

Fig.9 Equilibrium partial pressure and stable solid state phase in B-O-C system at 1600K
With the proceeding of these reactions, the partial pressure (p(CO)) near B4C powder decreases, CO in the environment diffuses onto B4C particle surface and makes the above reactions go on. At the same time, the formed B2O3(g) and BO(g) diffuse into environment and reacts with CO(p(CO)=1MPa):
B2O2(g)+CO(g)=B2O3(l)+C(s)(8)
BO(g)+(1/2)CO(g)=(1/2)B2O3(l)+
(1/2)C(s)(9)
Reactions (8) and (9) make CO reduced to carbon, and B2O3 exists in liquid phase; simultaneously, reaction (1) causes an expansion of the reacting system, which walls up pores. B2O3 liquid filters into pores through capillary, and carbon in pores more firmly combines with other carbon atoms in liquid through dissolution, separating out and sintering to form a solid solution, and the formed solid solution further walls up pores to prevent oxygen to filter. Material thus prepared has a more compact inner structure and of course, better anti-oxygenic property.
Within the temperature range of 9000-1200℃, SiO2 formed exists in solid state, B2O3 liquid, there is also a eutectic liquid of both which takes role of composite protecting film; among which, B2O3 and the eutectic liquid dominate in increasing anti-oxidation ability.
5 CONCLUSIONS
1) Powder mixture of green petroleum, B4Cand SiC, in certain proportion, after grinding and then with the adding of short carbon fiber, can prepare high mechanical strength C/C composites with excellent anti-oxidation property, that is, mass loss less than 1% within the temperature range from 900 to 1100℃. This binderless and direct model press process is also characterized by its simple processing procedure.
2) The excellent anti-oxidation property of the materials prepared in the above way is due to a) the solid SiO2 formed from SiC, which restrains the filtering of oxygen and also, its volume expansion brought about by the reaction takes roles both walling up the original pores and making the material more compact; b) the liquid B2O3 formed fom the reaction of B4C not only makes the combination with C, B4C and SiC tighter through forming solid solution, but also effects of equation (1) being an expansive process, which improves the microstructure of the material.
3) The anti-oxidation property indicated by the oxidation mass loss of C/C composites prepared with binderless process deteriorates with a) the increase of temperature at selected heating time and, b) the prolongation of oxidation time under certain temperature.
REFERENCES
[1]Christopher B. Influence of thermal properties on friction performance of carbon composites [J]. Carbon, 2001, 39: 1789-1801.
[2]ZHOU Sheng-mai, LIU Qi-cheng, et al. Influence of additives on oxidation resistance of binderless C/C composite [J]. Trans Nonferrous Met Soc China, 2003,13: 162-164.
[3]WANG Qing, LI Hong, et al. Monodispersed hard carbon spherules with uniform nanopores [J]. Carbon, 2001, 39: 2211-2214.
[4]LIU Yong-cai, XIU Xiao-ping. Methanol electro-oxidation on mesocarbon microbead supported cataly [J]. Carbon, 2002, 40: 2375-2380.
[5]SU Jun-ming, CUI Hong, LI Rui-zhen, et al. The structure and properties of new C/C composite [J]. New Carbon Materials(China), 2000, 15(2): 11-15.
[6]Dietrich G, Kienzle A, et al. Fiber-reinforced composite ceramice and method of producing the same [P]. US Patent 6261981, 2001.
[7]LIU Wen-chuan. The anti-oxidation C/C composites [J]. Carbon, 2000, 2: 23-35.
[8]YANG Jing, Iiegbusi O J. Kinetics of silicon-matal alloy infiltration into porous carbon [J]. Composites(Part A), 2000, 31: 617-625.
[9]HAN Hong-mei, ZHANG Xiu-lian, LI He-jun, et al. Mechanical behavior of C/C composites under high-temperature [J]. New Carbon Materials, 2003, 1: 20-25.
[10]GUO Quan-gui, SONG Jin-ren, LIU Lang, et al. Oxidation resistance of SiC-B4C/C composites with self-healing properties at high temperatures [J]. New Carbon Materials,1988, 3: 2-7.
[11]ZHANG Wei-gang, CHENG Hui-ming, SHEN Zu-hong, et al. The effects TiC additive on the oxidation behavior of C-SiC-B4C composites [J]. New Carbon Materials, 1988, 3: 8-13.
[12]ZHANG Wen-jie, LI Lang. Carbon Composites Refractory [M]. Beijing: Science Press, 1990. 24-51.(in Chinese)
[13]ZHANG Nian-dong. Technology of SiC Grinder [M]. Beijing: China Machine Press, 1982. 47-89.(in Chinese)
[14]Mckee D W. Chemistry and Physics of Carbon [M]. New York: Marcel Dekker Press, 1981. 18-20.
(Edited by PENG Chao-qun)
Foundation item: Projects(03WHY3018; 03JKY1018-2) supported by the Natural Science Foundation of Hunan Province
Received date: 2005-03-18; Accepted date: 2005-09-26
Correspondence: LIU Qi-cheng, Professor, PhD; Tel: +86-731-5204685; E-mail: liuqc1856@hotmail.com