纳米CeO2对电沉积Al/α-PbO2/β-PbO2镀层结构和性能的影响
来源期刊:中国有色金属学报(英文版)2013年第5期
论文作者:陈 阵 余 强 廖登辉 郭忠诚 武 剑
文章页码:1382 - 1389
关键词:稀土;CeO2;复合电极材料;α-PbO2;β-PbO2;槽电压;惰性阳极
Key words:rare earth; CeO2; composite electrode material; α-PbO2; β-PbO2; cell voltage; inert anode
摘 要:采用阳极氧化法制备掺杂稀土氧化物(CeO2)的 Al/α-PbO2/β-PbO2复合电极,考察CeO2的掺杂以及α-PbO2作为中间层对Al/α-PbO2/β-PbO2电极性能的影响。结果表明:α-PbO2作为中间层有利于β-PbO2的结晶,β-PbO2比α-PbO2更适合作电极表层;CeO2的掺杂能够改变晶粒尺寸和晶粒结构,提高电极的催化活性,并改变PbO2晶粒的沉积机理;掺杂CeO2的PbO2电极的电催化活性能得到有效提高,从而降低析氧电位和槽电压。
Abstract: Al/α-PbO2/β-PbO2 composite electrodes doped with rare earth oxide (CeO2) were prepared by anodic oxidation method investigate the influence of nano-CeO2 dopants on the properties of Al/α-PbO2/β-PbO2-CeO2 electrodes and the impact of α-PbO2 as the intermediate layer. The results show that using α-PbO2 as the intermediate layer will benefit the crystallization of β-PbO2 and β-PbO2 is more suitable as the surface layer than α-PbO2. CeO2 dopants change the crystallite size and crystal structure, enhance the catalytic activity, and even change the deposition mechanism of PbO2. The doping of CeO2 in the PbO2 electrodes can enhance the electro-catalytic activity, which is helpful for oxygen evolution, and therefore reduce the cell voltage.
Trans. Nonferrous Met. Soc. China 23(2013) 1382-1389
Zhen CHEN1, Qiang YU1, Deng-hui LIAO2, Zhong-cheng GUO2, Jian WU2
1. Faculty of Science, Kunming University of Science and Technology, Kunming 650093, China;
2. Faculty of Metallurgy and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China
Received 6 April 2012; accepted 10 September 2012
Abstract: Al/α-PbO2/β-PbO2 composite electrodes doped with rare earth oxide (CeO2) were prepared by anodic oxidation method investigate the influence of nano-CeO2 dopants on the properties of Al/α-PbO2/β-PbO2-CeO2 electrodes and the impact of α-PbO2 as the intermediate layer. The results show that using α-PbO2 as the intermediate layer will benefit the crystallization of β-PbO2 and β-PbO2 is more suitable as the surface layer than α-PbO2. CeO2 dopants change the crystallite size and crystal structure, enhance the catalytic activity, and even change the deposition mechanism of PbO2. The doping of CeO2 in the PbO2 electrodes can enhance the electro-catalytic activity, which is helpful for oxygen evolution, and therefore reduce the cell voltage.
Key words: rare earth; CeO2; composite electrode material; α-PbO2; β-PbO2; cell voltage; inert anode
1 Introduction
Studies on inert anode materials used in zinc-plating industry have mostly focused on conventional anode materials, such as lead-based alloys, dimensionally stable anode, Au, Pt and glassy carbon [1-3]. However, the disadvantages of conventional anode materials including high cell voltage, poisonousness, low mechanical strength, inefficient conductivity and impurity product caused by anode re-dissolve have always perplexed us [4,5]. Therefore, further researches have been undertaken to discover more efficient electrode materials and metal oxide-film electrodes, such as PbO2, SnO2 and RuO2 [6-10].
Lead dioxide (PbO2) electrode has been extensively used in electrochemical industry and regarded as an excellent metal oxide electrode because of its low price compared with noble metals, good chemical stability, and high catalytic activity for oxygen evolution. The application of PbO2 electrode is largely dependent upon its structure, morphology, and phase composition. In order to further improve the electrochemical properties of PbO2 toward various applications, incorporating some ions or particles such as rare earth into the film of lead oxide was investigated.
It is well known that PbO2 has two different crystallographic forms: orthorhombic and tetragonal (α and β). α-PbO2 is obtained from alkaline solution and β-PbO2 from acid solution [11]. α-PbO2 has a better contact between particles, and a more compact structure than β-PbO2. Unfortunately, more compact structure leads to bad conductivity compared with β-PbO2 [12]. Different electro-catalytic activities of α and β forms of PbO2 were observed in other studies. It was also observed that the structure of crystallization of PbO2 films influenced the electro-catalytic properties of the material [13-19].
A new type of PbO2 anode was widely used in electrolysis [20]. This electrode is made up of four layers: a metal base, a conductive layer (protecting the substrate from passivation), α-PbO2 as the intermediate layer, and β-PbO2 as surface layer. Aluminum is relatively cheap and has a good conductivity. The electrode material by electrodepositing lead dioxide on Al substrate has huge market prospects. Rare earth oxides, as powerful oxidants, can easily catalyze the oxidation of organics. It was reported that [21-24] metal oxides have been successfully doped into PbO2 electrodes by anodic codeposition. However, few researches on the doping of rare earth oxide into PbO2 electrodes have been reported.
In this work, α-PbO2 was chosen as intermediate layer, and β-PbO2 as the surface layer. Ce(IV) was introduced as a doping agent into PbO2 electrode for its superior properties [25] were successfully doped by the anodic codeposition method. It changes the crystallite size and enhances the catalytic activity in the oxidation of the material. Scanning electron microscopy (SEM), X-ray diffraction (XRD) and energy-dispersive spectroscopy (EDS) were used to examine the changes in the coating.
2 Experimental
2.1 Preparation of coatings
After a series of pretreatments: sand blasting→ degreasing (40 g/L Na3PO4, 10 g/L Na4SiO4, 3 min)→ chemically etching (10 mL/L HF, 50 mL/L HNO3, 90 s) → coating by a conductive layer, 1060# Al was used as an anode and machined to dimensions of 40 mm×10 mm×1 mm. Pure lead was used as a cathode. The electrodeposition of α-PbO2 was conducted in an alkaline bath, while that of β-PbO2 was conducted in an aqueous bath. The plating conditions are listed in Table 1 and Table 2, respectively. Two types of anodes were made: one was Al/conductive layer/β-PbO2 (No. 1), and the other was Al/conductive layer/α-PbO2/β-PbO2 (No. 2). The difference between them was the intermediate layer α-PbO2. CeO2 was introduced as a doping agent into the aqueous β-PbO2 electrolyte. By the anodic codeposition method, CeO2 was successfully doped into No. 2 electrode.
2.2 Measuring instruments
Micrographs of the coating surface were obtained by scanning electron microscopy (ESEM, FEI, Quanta200). The structures of the films were analyzed by X-ray diffraction (XRD) with Co Kα radiation in a standard X-ray diffract meter (Rigaku D/max-1200X). The element contents were tested by energy-dispersive spectroscopy (EDAX-Phoenix). The over-potential was tested in 1.3 mol/L ZnSO4+1 mol/L H2SO4 (pH=4.5) using polarization curves at a scanning rate of 50 mV/s. (CHI660D, Chenhua, China). During the measurement, a three-electrode system was used. The working electrode was the composite coating (1 cm×1 cm). The reference electrode was saturated calomel electrode (SCE) used directly in contact with the working solution. The counter electrode was platinum (1 cm×1 cm).The measurement was conducted at room temperature.
3 Results and discussion
3.1 Choice of intermediate layer
In this work, α-PbO2 was obtained at a constant current density of 20 mA/cm2 for 30 min in the solution containing 180 g/L NaOH saturated with PbO(s), and was used as the intermediate layer. A typical XRD pattern of α-PbO2 deposited on Al/conductive layer is shown in Fig. 1. The surface morphology of α-PbO2 coating is shown in Fig. 2. The XRD pattern indicates the presence of α-PbO2. The characteristic peaks are α-PbO2 (scrutinyite), and β-PbO2 (plattnerite) is not observed. The grain of α-PbO2 looks like rod or fiber. Numerous crystal edges and a few pores are found in such coating. However, these defects are proved to be helpful for the deposition of β-PbO2 in further study.
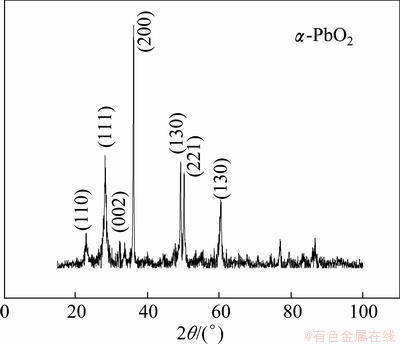
Fig. 1 XRD pattern of α-PbO2 (scrutinyite)
Table 1 Deposition condition for α-PbO2 coatings

Table 2 Deposition condition for β-PbO2 coatings


Fig. 2 SEM image of α-PbO2 coating (scrutinyite)
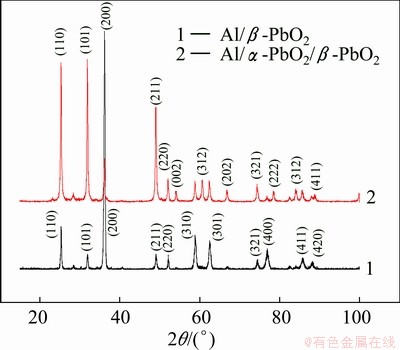
Fig. 3 XRD patterns of β-PbO2 coatings
The XRD patterns of two types of β-PbO2 coatings (No. 1 and 2) shown in Fig. 3 all accord with the PDF#41—1492, which is plattnerite (β-PbO2). No. 1 was prepared directly on the Al conductive layer, while No. 2 was prepared on the α-PbO2 layer. The main crystal plane of No. 1 is (200), while that of No. 2 is (101). The main crystal plane of No. 2 is consistent with the results reported by ABACI et al [26]. Furthermore, No. 2 has more peaks: (002), (312), (202), (222), (312), and they are all consistent with the PDF#41—1492, showing that No. 2 is better crystallized. Apparently, α-PbO2 as intermediate layer will improve the crystallization of β-PbO2.
Table 3 Reactant and product of anodic process and  [27,28]
[27,28]
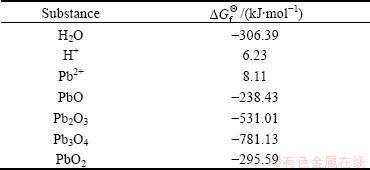
Table 4 Reaction of anodic process and 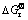 [27,28]
[27,28]
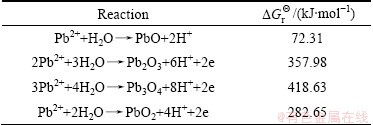
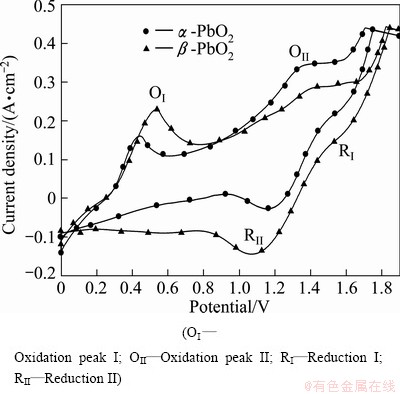
Fig. 4 CV curves of α-PbO2and β-PbO2 coatings
Table 3 shows the 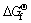 values of several substances, which may participate as a reactant or product in producing PbO2. Table 4 presents the
values of several substances, which may participate as a reactant or product in producing PbO2. Table 4 presents the 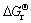 of each reaction and demonstrates that thermodynamic reaction followed by ease of PbO>PbO2>Pb2O3>Pb3O4. Figure 4 shows that the precipitation potential of α-PbO2 is lower than that of β-PbO2, which requires small electromotive force (E). So, according to the equation: ΔG=-nEF (ΔG>0), when the product is α-PbO2, the ΔG is low, the energy barrier is smaller and therefore the thermodynamic stability is poor. Researchers also conducted a study on this issue and came to conclusions that the thermodynamic stability of β-PbO2 is better than that of α-PbO2 [29]. Above all, the thermodynamic reaction (precipitation potential) followed by ease of PbO>α-PbO2>β-PbO2>Pb2O3>Pb3O4. In this case, β-PbO2 coating is more suitable as a surface anode materials.
of each reaction and demonstrates that thermodynamic reaction followed by ease of PbO>PbO2>Pb2O3>Pb3O4. Figure 4 shows that the precipitation potential of α-PbO2 is lower than that of β-PbO2, which requires small electromotive force (E). So, according to the equation: ΔG=-nEF (ΔG>0), when the product is α-PbO2, the ΔG is low, the energy barrier is smaller and therefore the thermodynamic stability is poor. Researchers also conducted a study on this issue and came to conclusions that the thermodynamic stability of β-PbO2 is better than that of α-PbO2 [29]. Above all, the thermodynamic reaction (precipitation potential) followed by ease of PbO>α-PbO2>β-PbO2>Pb2O3>Pb3O4. In this case, β-PbO2 coating is more suitable as a surface anode materials.
Figure 5(a) presents the β-PbO2 prepared directly on the conductive layer (βD), while Fig. 5(b) presents β-PbO2 prepared on α-PbO2 layer (αβ). The morphology of the βD shown in Fig. 5(a) looks like a nappe sheet, whereas that of αβ looks like a typical pyramid and the average grain size is 25 μm. It is obvious that coatings prepared on the α layer are more compact and uniform. Studies show that [30-32], α-PbO2 intermediate layer can effectively increase the firmness of the lead dioxide coating and the substrate, and the ease of the distortion of electrodeposition, and make the surface layer β-PbO2 distribute more uniformly. Therefore, new type of PbO2 electrode is composed of α-PbO2 intermediate layer and β-PbO2 surface layer, so a better catalytic activity is obtained [33].
3.2 CeO2 as doping agent
The compositional analysis of Al/α-PbO2/β-PbO2 electrodeposits was performed under different CeO2 concentrations in bath using energy dispersive spectroscopy. EDS spectra of β-PbO2-CeO2 films deposited under different conditions are shown in Fig. 6. It is found from Fig. 6 that with increasing the CeO2 concentration in bath from 10 to 50 g/L, Ce contents are 2.97%, 2.90%, 3.47%, 3.44% and 3.73%, respectively.
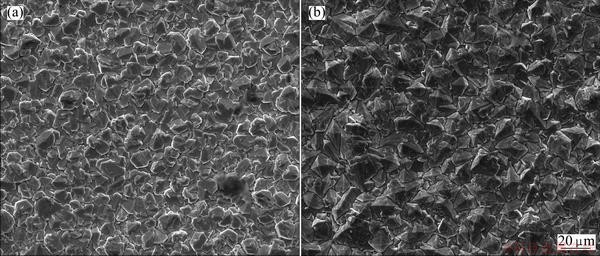
Fig. 5 SEM images of β-PbO2 prepared directly on conductive layer (a) and on α-PbO2 layer (b)
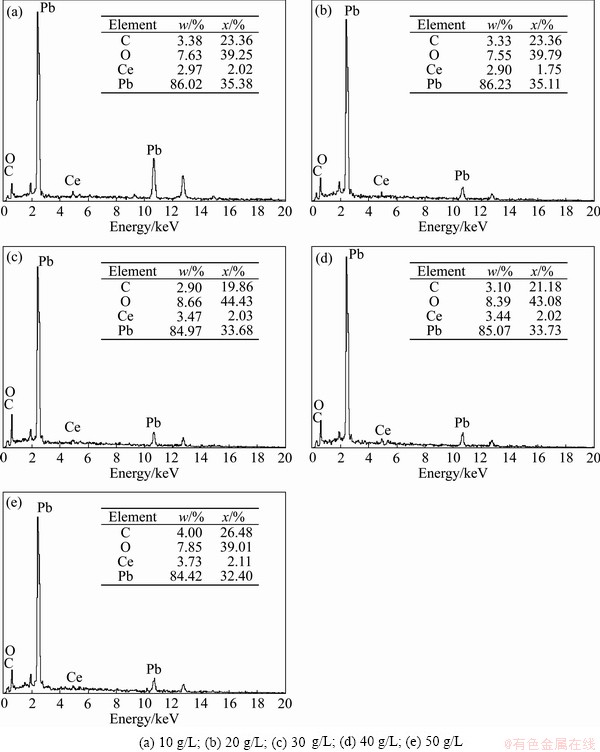
Fig. 6 EDS spectra of β-PbO2-CeO2 coatings with different concentrations of CeO2
Obviously, particles will get more opportunity to be embed into Al/α-PbO2/β-PbO2 composite by adding more nucleation points on the anodic surface. More particles suspending in the electrolyte are good for CeO2 to stay, absorb and form nucleation points. As a result, increasing the CeO2 concentration will surely help the co-deposition. Rare earth oxides can easily catalyze the oxidation of organics and change the surface morphology.
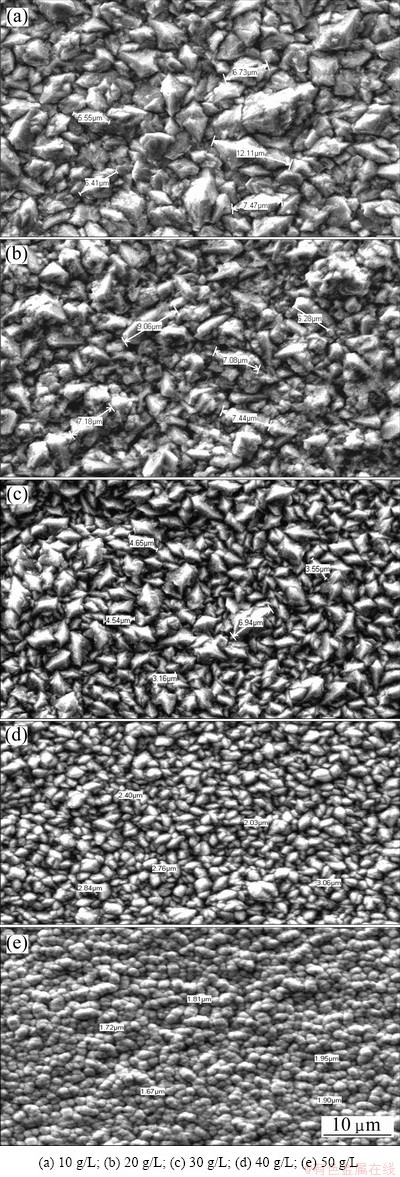
Fig. 7 SEM images of β-PbO2 with different concentrations of CeO2
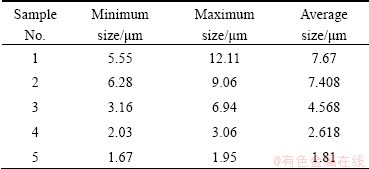
When ceria dioxide is used, as shown in Fig. 7, the surface morphology presents nodular shape. The average grain sizes decrease with the increase of Ce content. The average grain sizes are shown in Table 5. The present experimental results indicate that the crystallization process of PbO2 can be affected by adulteration of CeO2, thus changing surface microstructure of the coating. The average grain size of PbO2 decreases with the adulteration of CeO2 because the doped ceria dioxide provides a new center for PbO2 to nucleate, which hinders the further growth of PbO2. In other words, the nucleation rate is faster than the growing rate [34].
Table 5 Grain size of coatings
3.3 Over potential and catalytic activity for oxygen evolution
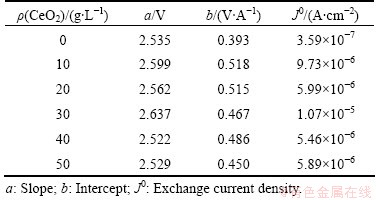
In zinc plating industry, anodic over potential, mainly caused by oxygen evolution reaction, will consume a large percentage of cell voltage. Therefore, a low oxygen evolution potential will save electricity and reduce the cell voltage a lot. Figure 8 gives the polarization curves of Al/α-PbO2/β-PbO2-CeO2 and Al/β-PbO2 electrodes in 1.3 mol/L ZnSO4+1 mol/L H2SO4 (pH=4.5). Compared with Al/α-PbO2/β-PbO2, adulteration of CeO2 can apparently lower the over-potential of PbO2. According to the Tafel equation:

where β is the transfer coefficient and J0 is the exchange current density, dividing the intercept of linear by its slope, J0 can be obtained, and the fitting curves are shown in Fig. 9. The exchange current densities are listed in Table 6, confirming that Al/α-PbO2/β-PbO2-CeO2 has a higher catalytic activity for oxygen evolution than Al/β-PbO2. Moreover, adulteration of CeO2 can greatly influence the exchange current. When CeO2 concentra- tion in bath is 30 g/L, the exchange current of Al/α-PbO2/β-PbO2-CeO2 electrode is the highest, indicating that the content of Ce can easily catalyze the oxidation of organics. On the other hand, the surface structure of PbO2 has a strong influence on the catalytic activity for oxygen evolution [35-37]. The SEM images of Al/α-PbO2/β-PbO2-CeO2 and Al/α-PbO2/β-PbO2 electrode show that the morphologies of the two types of electrodes are rather different. The surface of Al/α-PbO2/ β-PbO2 electrode presents many micro-cracks, and looks like a typical pyramid shape; the grains are more dispersive and large. Al/α-PbO2/β-PbO2-CeO2 is quite compact, and the grain size decreases. Therefore, the active surface area of Al/α-PbO2/β-PbO2-CeO2 electrode is larger than that of Al/β-PbO2. Moreover, electronic conduction exists in the rare earth oxides [38]. This demonstrates that ceria dioxide, as the catalytic sites, could strengthen the interface electron transfer of the electrode.
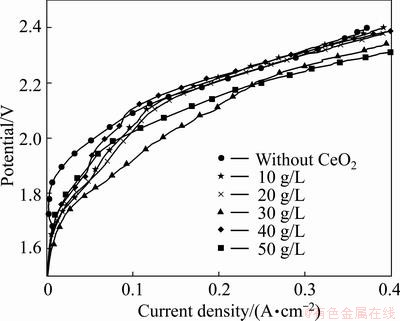
Fig. 8 Polarization curves of PbO2 electrodes prepared with different concentrations of CeO2 in bath
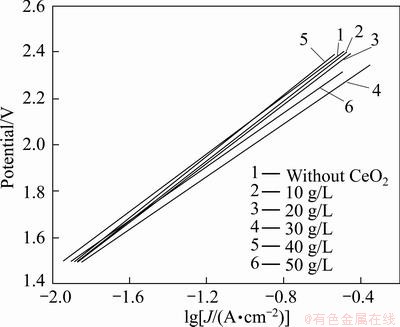
Fig. 9 Linear fitting of polarization curves of PbO2 electrodes prepared with different concentrations of CeO2 in bath
Table 6 Kinetic parameters of oxygen evolution
3.4 AC impedance
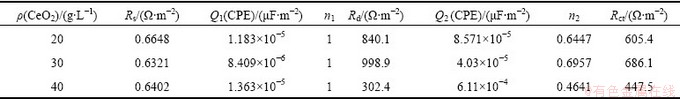
Figure 10 shows the Nyquist plots of the coatings prepared under different CeO2 concentrations. It can be seen that when CeO2 concentrations are 10 and 50 g/L, the plots are not the same as the others. Apparently, CeO2 as a doping agent changes the reaction mechanism. Coatings, which were prepared under CeO2 concentrations of 10 and 50 g/L, are coarse or can easily fall off. Therefore, only the other three were computed and analyzed. When CeO2 concentration is 20-40 g/L, polarization resistance (Rp) becomes large first and then gets small. When the fitting software (ZSimpWin) is used to fit and interpret the Nyquist plots, the obtained equivalent circuit is shown in Fig. 11 and the impedance fitting values are listed in Table 7. The equivalent circuit is composed of a solution resistance (Rs), two constant phase elements Q (CPE) which represent the coating capacitance and the double layer capacitance, a coating resistance and a charge transfer resistance (Rct). Generally, CPE/Q may exist because of the rough surface or uneven distribution of the electric field [39]. Table 7 shows that the values of n1 are all equal to 1, indicating that dispersion effect does not occur between the solution/coating layers. While values of n2 are all not equal to 1, indicating that dispersion effect occurs under the coating layer, or maybe occurs in electric double layer. When the value of n2 is closer to 1, the coating layer is smoother and dispersion effect is weaker. When CeO2 concentration is 30 g/L, dispersion effect of the coating is weaker in that n2 is maximum, which demonstrates a smoother surface. It is considered that in the low-frequency area, the corrosion resistance of the composite coatings can be reflected by the impedance. Such impedance corresponds to the the charge transfer resistance (Rct). Rct of the three coatings is not very different, only about 100 Ω/cm2. As the same as the dispersion effect, the largest Rct is tested on the coating when CeO2 concentration is 30 g/L. This is probably because CeO2 particles are firstly absorbed on the coating surface, by lowing the reaction area, formation of the oxidation and hydrated layer was hindered, and therefore Rct increases. When CeO2 particles are wrapped into the metal composite, the reaction area or active area is exposed again, oxidation and hydration occur, and then Rct decreases. This is probable why coatings will have different mechanisms when prepared with different CeO2 concentrations. Particles experience a complex process, including adsorption→desorption→inlay→ coating. So the composite deposition process is actually complicated.
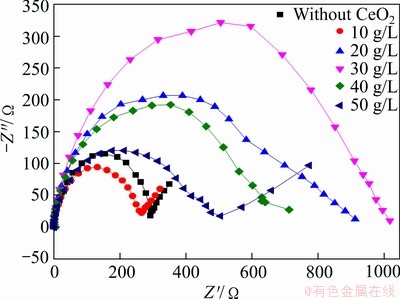
Fig. 10 Nyquist plots of coatings with different CeO2 concentrations
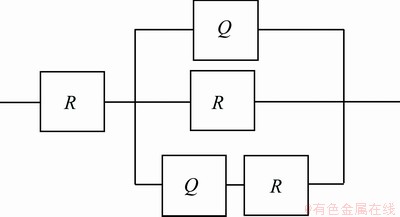
Fig. 11 Equivalent circuit of composite coatings in zinc plating solution
Table 7 Fitting parameters of equivalent circuit of electrode
4 Conclusions
1) α-PbO2 as intermediate layer can improve the deposition of β-PbO2, including structure and crystal plane. β-PbO2 has better thermodynamic stability, and is more suitable as a surface layer.
2) Rare earth oxide dopants can decrease the grain size of PbO2 and enlarge the active surface area and therefore change the content of Ce element in the coating.
3) The change of catalytic activity is mainly due to the specific role of the doped ceria dioxide and the different structures of electrodes.
References
[1] ZHI G Y, HUI M M, DONG B S. New degradation mechanism of Ti/IrO2+MnO2 anode for oxygen evolution in 0.5M H2SO4 solution [J]. Electrochimica Acta, 2008, 53(18): 5639-5643.
[2] PANICA V V, JOVANOVICA V M, TERZICA S I , BARSOUMB M W, JOVICC V D, DEKANSKIA A B. The properties of electroactive ruthenium oxide coatings supported by titanium-based ternary carbides [J]. Surface and Coatings Technology, 2007, 202: 319-324.
[3] AROMAA J, FORSEN O. Evaluation of the electrochemical activity of a Ti-RuO2-TiO2 permanent anode [J]. Electrochimica Acta, 2006, 51(27): 6104-6110.
[4] ZHAO Q, LIU Y, MULLER-STEINHAGEN H, LIU G. Graded Ni-P-PTFE coatings and their potential applications [J]. Surface and Coatings Technology, 2002,155: 279-284.
[5] FENG Q Y, LI T J, JIN J Z. Research on the mechanism of composite electroplating and its latest progress rare metal materials and engineering [J]. Rare Metal Materials and Engineering, 2007, 36(3): 559-564. (in Chinese)
[6] BIRAME B, ENRIC B, BEATRICE M, PIERRE ALAIN M, CHRISTOS C, GIUSEPPE F, GIANCARL S. Electrochemical incineration of chloromethylphenoxy herbicides in acid medium by anodic oxidation with boron-doped diamond electrode [J]. Electrochim Acta, 2006, 51(15): 2872-2880.
[7] CARLOS A M H, MARCO A Q, CHRISTOS C, SERGIO F, ACHILLE D B. Electrochemical incineration of chloranilic acid using Ti/IrO2, Pb/PbO2 and Si/BDD electrodes [J]. Electrochim Acta, 2004, 50(4): 949-956.
[8] CARMEM L P S Z, PIERRE-ALAN M, CHRISTOS C, ADALGISA R, DE A, JULIEN F C B. Electrochemical oxidation of p-chlorophenol on SnO2–Sb2O5 based anodes for wastewater treatment [J]. Journal of Applied Electrochemistry, 2003, 33(12): 1211-1215.
[9] PANIC V V, DEKANSKI A B, VIDAKOVIC T R, MISKOVIC- STANKOVIC V B, JAVANOOVIC B Z, NIKOLIC V Z. Oxidation of phenol on RuO2–TiO 2/Ti anodes [J]. Solid State Electrochem, 2005, 9(1): 43-54.
[10] WANG Y H, CHENG S, CHAN K Y, LI X Y. Electrolytic generation of ozone on antimony- and nickel-doped tin oxide electrode [J]. Journal of The Electrochemical Society, 2005, 152(11): D197-D200.
[11] CARR J P, HAMPSON N A. The lead dioxide electrode [J]. Chemical Reviews, 1972, 72(6): 679-702.
[12] PETERSSON I, AHLBERG E, BERGHULT B. Parameters influencing the ratio between electrochemically formed α and β-PbO2 [J]. Journal of Power Sources, 1998, 76(1): 98-105.
[13] AMADELLI R, MALDOTTI A, MOLINARI A, DANILOV F I, VELICHENKO A B. Influence of the electrode history and effects of the electrolyte composition and temperature on O2 evolution at β-PbO2 anodes in acid media [J]. Journal of Electroanalytical Chemistry, 2002, 534(1): 1-12.
[14] PAVLOV D, MONAHOV B. Mechanism of the elementary electrochemical processes taking place during oxygen evolution on the lead dioxide electrode [J]. Journal of The Electrochemical Society, 1996, 143(11): 3616-3629.
[15] MUNICHANDRAIA N, SATHYANARAYANA S. Insoluble anode of α-lead dioxide coated on titanium for electrosynthesis of sodium perchlorate [J]. Journal of Applied Electrochemistry, 1988, 18(2): 314-316.
[16] ABACI S, PEKMEZ K, PEKMEZ P, YILDIZ A. Electrocatalysis of polyaniline formation by PbO2 in acetonitrile [J]. Journal of Applied Polymer Science, 2003, 87(4): 599-605.
[17] ABACI S, TAMER U, PEKMEZ K, YILDIZ A. Electrosynthesis of 4,4′-dinitroazobenzene on PbO2 electrodes [J]. Journal of Applied Electrochemistry, 2002, 32(2): 193-196.
[18] ABACI S, YILDIZ A. Electropolymerization of thiophene and 3-methylthiophene on PbO2 electrodes [J]. Journal of Electroanalytical Chemistry, 2004, 569(1): 161-168.
[19] VELICHENKO A B, AMADELLI R, BENEDETTI A, GIRENKO D V, KOVALYOV S V, DANILOVA F I. Electrosynthesis and physicochemical properties of PbO2 films [J]. Journal of The Electrochemical Society, 2002, 149(9): C445-C449.
[20] UEDA M, WATANABE A, KAMEYAMA T, MATSUMOTO Y, SEKIMOTO M, SHIMAMUNE T. Performance characteristics of a new type of lead dioxide-coated titanium anode [J]. Journal of Applied Electrochemistry, 1995, 25: 817-822.
[21] CATTARIN S, GUERRIERO P, MUSIANI M. Preparation of anodes for oxygen evolution by electrodeposition of composite Pb and Co oxides [J]. Electrochim Acta, 2001, 46(26-27): 4229-4234.
[22] CATTARIN S, FRATEUR I, GUERRIERO P, MUSIANI M. Electrodeposition of PbO2+CoOx composites by simultaneous oxidation of Pb2+ and Co2+ and their use as anodes for O2 evolution [J]. Electrochim Acta, 2000, 45: 2279-2288.
[23] MUSIANI M, GUERRIERO P. Oxygen evolution reaction at composite anodes containing Co3O4 particles [J]. Electrochim Acta, 1998, 44(8-9): 1499-1507.
[24] MUSIANI M, FURLANETTO F, GUERRIEROP. Electrochemical deposition and properties of PbO2+Co3O4 composites [J]. Journal of Electroanalytical Chemistry, 1997, 440(1-2): 131-138.
[25] SRIVASTAVA M, WILLIAM GRIPS V K, RAJAM K S. Electrodeposition of Ni-Co composites containing nano-CeO2 and their structure, properties [J]. Applied Surface Science, 2010, 257(3): 717-722.
[26] ABACI S, PEKMEZ K, HOKELEK T, YILDIZ A. Investigation of some parameters influencing electrocrystallisation of PbO2 [J]. Journal of Power Sources, 2000, 88(2): 232-236.
[27] LI Di. Electrochemical principle [M]. Beijing: Beihang University Press, 1999. (in Chinese)
[28] LI Wen-chao. Metallurgical and materials physical chemistry [M]. Beijing: Metallurgical Industry Press, 2001. (in Chinese)
[29] ZHOU Ya-ning, WAN Ya-zhen, LIU Jin-dun. Preparation and application of PbO2 electrode [J]. Salt Industry, 2006, 38(10): 8-11. (in Chinese)
[30] WENG Feng, YU Bin. Preliminary research of determining COD using Pt/PbO2 electrode [J]. Journal of Nanjing University of Technology, 2002, 24(2): 93-96.
[31] PETERSSON I, AHLBERG E, BERGHULT B. Parameters influencing the ratio between electro-chemically formed α and β-PbO2 [J]. Journal of Power Sources, 1988, 76(1): 98-105.
[32] FUKASAWA A. Production of commercial large lead dioxide electrode-application of a new-type lead dioxide electrode to electrolytic refining [J]. Mining and Metallurgical Institute of Japan, 1984, 1157: 599-602.
[33] ZHANG Zhao-xian. Titanium electrode industry [M]. Beijing: Metallurgical Industry Press, 2000. (in Chinese)
[34] CASELLATO U, CATTARIN S, MUSIANI M. Preparation of porous PbO2 electrodes by electrochemical deposition of composites [J]. Electrochim Acta, 2003, 48(27): 3991-3998.
[35] AMADELLI R, ARMELAO L, VELICHENKO A B, NIKOLENKO N V, GIRENKO D V, KOVALYOV S V, DANILOV F I. Oxygen and ozone evolution at fluoride modified lead dioxide electrodes [J]. Electrochim Acta, 1999, 45: 713-720.
[36] ABACI S, PEKMEZ K, HOKELEK, YILDIZ A. Investigation of some parameters influencing electrocrystallisation of PbO2 [J]. Journal of Power Sources, 2000, 88(2): 232-236.
[37] HO J C K, TREMILIOSI G, SIMPRAGA R, CONWAY B E, Structure influence on electrocatalysis and adsorption of intermediates in the anodic O2 evolution at dimorphic alpha-PbO2 and beta-PbO2 [J]. Journal of Electroanalytical Chemistry, 1994, 366(5):147-162.
[38] MINACHEV F M. The application of rare earth for catalyst [M]. Beijing: Science Press, 1987. (in Chinese)
[39] LU Chang-feng, LU Min-xu, ZHAO Guo-xian, BAI Zhen-quan, YAN Mi-lin, YANG Yan-qing. The analysis of electrode reactions of CO2 corrosion of N80 steel [J]. Acta Metallurgica Sinica, 2002, 38(7): 770-774. (in Chinese).
陈 阵1,余 强1,廖登辉2,郭忠诚2,武 剑2
1. 昆明理工大学 理学院,昆明 650093;
2. 昆明理工大学 冶金与能源工程学院,昆明 650093
摘 要:采用阳极氧化法制备掺杂稀土氧化物(CeO2)的 Al/α-PbO2/β-PbO2复合电极,考察CeO2的掺杂以及α-PbO2作为中间层对Al/α-PbO2/β-PbO2电极性能的影响。结果表明:α-PbO2作为中间层有利于β-PbO2的结晶,β-PbO2比α-PbO2更适合作电极表层;CeO2的掺杂能够改变晶粒尺寸和晶粒结构,提高电极的催化活性,并改变PbO2晶粒的沉积机理;掺杂CeO2的PbO2电极的电催化活性能得到有效提高,从而降低析氧电位和槽电压。
关键词:稀土;CeO2;复合电极材料;α-PbO2;β-PbO2;槽电压;惰性阳极
(Edited by Xiang-qun LI)
Foundation item: Project (50964008) supported by the National Natural Science Foundation of China; Project (2010287) supported by Analysis and Testing Foundation of Kunming University of Science and Technology, China
Corresponding author: Qiang YU; Tel: +86-13708460246; E-mail: yuqiang0015@163.com
DOI: 10.1016/S1003-6326(13)62607-2

