文章编号:1004-0609(2014)10-2652-06
铝酸钠溶液中草酸钠溶解度计算模型的建立及应用
李小斌1, 2,徐 旺1, 2,张玉通1, 2,齐天贵1, 2
(1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 难冶有色金属资源高效利用国家工程实验室,长沙 410083)
摘 要:应用Bromley方程,结合草酸钠溶解热力学理论及其在水溶液、氢氧化钠溶液中的溶解度数据,得出草酸钠的Bromley参数为-0.045,并以此为基础建立铝酸钠溶液中草酸钠的溶解度计算模型。应用该模型计算纯铝酸钠溶液体系中草酸钠的溶解度,结果与文献数据吻合较好。在此基础上,模拟计算了拜耳法氧化铝生产过程中草酸钠平衡浓度的变化规律。结果表明:碱浓度越高、温度越低、苛性比越高,铝酸钠溶液中草酸钠平衡浓度越低;铝酸钠溶液体系中,碳酸钠、硫酸钠对草酸钠溶解度的影响很小。这些结果可以解释草酸钠在生产氧化铝过程中的积累和析出规律,有助于生产过程铝酸钠溶液中草酸钠含量的控制。
关键词:草酸钠;溶解度;计算模型;铝酸钠溶液
中图分类号:TF821 文献标志码:A
Establishment and application of calculation model of sodium oxalate solubility in sodium aluminate solution
LI Xiao-bin1, 2, XU Wang1, 2, ZHANG Yu-tong1, 2, QI Tian-gui1, 2
(1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. National Engineering Laboratory for Efficient Utilization of Refractory Non-ferrous Metals Resources,
Central South University, Changsha 410083, China)
Abstract: The calculation model of sodium oxalate’s solubility in sodium aluminate solution was established by Bromley equation with the solubility values of sodium oxalate in the aqueous and sodium hydroxide solution, and Bromley parameter of sodium oxalate calculated by this model is -0.045. Using this calculation model, the solubility values of sodium oxalate in sodium aluminate solution were calculated and a high degree of agreement can be reached compared with the data in literatures. On this basis, the equilibrium concentration values of sodium oxalate in Bayer liquors were calculated. The results show that, the higher the alkali concentration and caustic ratio are or the lower the temperature is, the lower the equilibrium concentration of sodium oxalate is. The concentration of sodium carbonate or sodium sulfate has little effect on the solubility of sodium oxalate. These results can explain the law of the accumulation and precipitation of sodium oxalate in alumina production process, and contribute to controlling the concentration of sodium oxalate in the process.
Key words: sodium oxalate; solubility; calculation model; sodium aluminate solution
拜耳法氧化铝生产过程中,多种有机物通过氧化分解并与NaOH反应生成草酸钠,草酸钠随着溶液的循环而不断积累,直至过饱和析出,从而影响生产的稳定运行和产品质量[1-2],尤其使晶种分解得到的产品严重细化。在铝酸钠溶液中加入有机添加剂,以抑制草酸根对种分过程产生的不利影响是工业上常用的手段。尹周澜等[3]认为有机添加剂抑制草酸钠的不利影响、强化铝酸钠溶液的分解是通过有效吸附到晶种表面、减少甚至消除草酸离子在晶种表面的吸附、从而使铝酸根离子靠近晶种表面等机理来进行的。但是,在铝酸钠溶液循环过程中,大量草酸钠积累时,有机添加剂的抑制作用十分有限,因而,从生产系统中排除草酸盐是解决其对生产影响的根本措施。已研究的脱除草酸钠的方法主要有结晶析出法[4-5]、吸附法[6-7]、化学沉淀法等[8-9],这些方法的效率均与铝酸钠溶液中草酸钠的浓度紧密相关,因此,研究草酸钠在铝酸钠溶液中的溶解度具有重要意义。
有关草酸钠在铝酸钠溶液中的溶解度主要通过实验测定。THE等[10]对草酸钠的表观溶解度实验数据进行多元回归分析后,确定了工业铝酸钠溶液中草酸钠表观溶解度与温度、全碱浓度之间的关系;POWER等[11]研究了有机物存在时,草酸钠在铝酸钠溶液中的溶解度。但由于草酸钠在铝酸钠溶液中存在明显的过饱和现象,影响因素复杂多变,难以通过实验测定获得大量的、准确的数据,因而有人试图通过理论计算确定其溶解度。GILBERT等[12]建立种分作业条件下草酸钠溶解度的物理化学模型,该模型可计算在较宽的Na2Ok浓度范围内草酸钠的平衡浓度。事实上,由于铝酸钠溶液中离子之间、离子与溶剂之间的相互作用较为复杂,上述模型在计算离子活度系数时进行了简化处理,其应用具有一定的局限性。REYNOLDS[13]以草酸钠为例,设计一种计算物质溶解度的混合模型,但由于铝酸钠溶液具有不同于常规溶液的过饱和性,该模型并不适用于计算草酸钠在铝酸钠溶液中的溶解度计算。本文作者基于Bromley方程浓度的适用范围较宽,可用于多组分电解质水溶液体系等特点[14-15],提出了一种新的计算铝酸钠溶液中草酸钠溶解度的方法并建立计算模型,以期为预测不同铝酸钠溶液体系中草酸钠的溶解度奠定理论基础。
1 计算模型的建立
1.1 草酸钠溶解热力学基础
纯水及碱性体系中,对于草酸钠的溶解反应:
 (1)
(1)
在平衡条件下有
 (2)
(2)
式中:K为反应的平衡常数; 为草酸钠的平均活度因子;
为草酸钠的平均活度因子;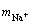 和
和 分别为相应离子的质量摩尔浓度。
分别为相应离子的质量摩尔浓度。
根据Bromley方程[16-18],草酸钠平均活度因子 表达式可以写为
表达式可以写为
 (3)
(3)
式中:


式中:Am为Debye-Hückel理论常数;I为溶液的离子强度;N代表Na+,C代表 ;zN和zC分别代表Na+和
;zN和zC分别代表Na+和 的价态;na(c)和j(k)分别表示溶液中能与Na+(
的价态;na(c)和j(k)分别表示溶液中能与Na+( )结合的总的阴(阳)离子种类数和第j(k)种阴(阳)离子;zj代表溶液中能够与Na+结合的第j种阴离子的价态;zk代表溶液中能够与
)结合的总的阴(阳)离子种类数和第j(k)种阴(阳)离子;zj代表溶液中能够与Na+结合的第j种阴离子的价态;zk代表溶液中能够与 结合的第k种阳离子的价态;BNj、BkC分别为电解质Nj、kC的Bromley参数;mj(k) 为第j(k)种阴(阳)离子的质量摩尔浓度;
结合的第k种阳离子的价态;BNj、BkC分别为电解质Nj、kC的Bromley参数;mj(k) 为第j(k)种阴(阳)离子的质量摩尔浓度; 和
和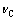 分别代表Na+和
分别代表Na+和 的计量数。
的计量数。
结合式(2)可知建立计算模型的关键为求得K及 的值。
的值。
1.2 模型参数的确定
鉴于草酸钠在纯水和氢氧化钠溶液中的溶解度数据比较充分,可根据其在两种溶液中的溶解度数据求 。BATELLA等[19]测定的不同温度下草酸钠在水中的溶解度如表1所列,表2则表示DONALD等[20]测得的不同温度下草酸钠在氢氧化钠溶液中的溶解度。
。BATELLA等[19]测定的不同温度下草酸钠在水中的溶解度如表1所列,表2则表示DONALD等[20]测得的不同温度下草酸钠在氢氧化钠溶液中的溶解度。
根据上述Bromley方程,对于纯的草酸钠水溶液,草酸钠的活度系数计算公式可整理为

 (4)
(4)
表1 草酸钠在纯水中的溶解度
Table 1 Solubility of sodium oxalate in pure water
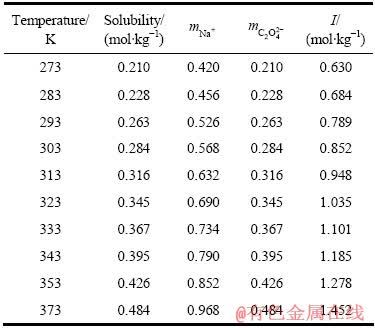
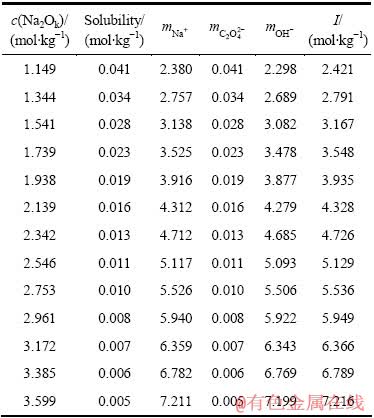
表2 333 K时不同碱浓度条件下草酸钠在NaOH溶液中的溶解度
Table 2 Solubility of sodium oxalate in NaOH solution with different alkali concentrations at 333 K
对于NaOH-Na2C2O4-H2O体系,则为
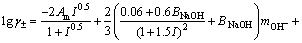
 (5)
(5)
根据质量作用定理,反应的平衡常数K仅与温度有关,而与其所处溶液的浓度无关,即在同一温度下,草酸钠在不同溶液中达到溶解平衡时的溶解反应平衡常数K相同。联立式(2)、(4)和(5),即可计算出相应的 ,其中
,其中 =0.0759[21]。计算得到的
=0.0759[21]。计算得到的 为-0.045,将得到的
为-0.045,将得到的 代入式(2)和(5),即可计算出不同温度下的平衡常数K,如表3所列。
代入式(2)和(5),即可计算出不同温度下的平衡常数K,如表3所列。
在K、 、
、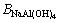 (
(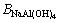 =0.0188[21])、
=0.0188[21])、 已知时,联立式(2)和(3)即可确定铝酸钠溶液体系中草酸钠的溶解度计算模型。
已知时,联立式(2)和(3)即可确定铝酸钠溶液体系中草酸钠的溶解度计算模型。
表3 不同温度下草酸钠溶解反应的平衡常数K
Table 3 Equilibrium constant (K) of sodium oxalate of dissolved reaction at different temperatures

2 计算模型的验证
2.1 NaOH-Na2C2O4-H2O体系中的模型验证
利用上述模型计算出303 K和368 K下NaOH-Na2C2O4-H2O体系中草酸钠的溶解度,与文献[19]数据的对比如图1所示。

图1 计算模型在NaOH-Na2C2O4-H2O体系的验证
Fig. 1 Verification of calculation model in NaOH-Na2C2O4- H2O system
由图1可知,在NaOH-Na2C2O4-H2O体系中,368 K时,由Bromley模型计算的草酸钠的溶解度与文献数据吻合程度极高。303 K时,计算值在氢氧化钠浓度较高时(>140 g/L)与文献值较吻合,在氢氧化钠浓度较低时(<140 g/L)有一定差别,但差别较小。由此可见,草酸钠Bromley参数取值合理。
2.2 NaOH-NaAl(OH)4-Na2C2O4-H2O体系中的模型验证
在NaOH-NaAl(OH)4-Na2C2O4-H2O体系中,依据上述模型计算出不同温度、不同浓度条件下,草酸钠在铝酸钠溶液中的溶解度如图2所示。
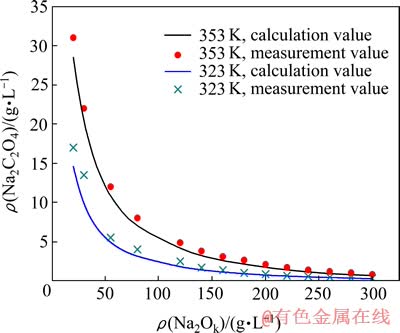
图2 计算模型在NaOH-NaAl(OH)4-Na2C2O4-H2O体系的验证(αk=3)
Fig. 2 Verification of calculation model in NaOH-NaAl(OH)4- Na2C2O4-H2O system (αk=3)
由图2可知,理论计算数据与实验数据[10]相比,吻合程度很高,说明利用该模型能够有效地预测铝酸钠溶液中草酸钠的溶解度。同时,Na2Ok浓度及温度对草酸钠的溶解度影响极大。Na2Ok浓度相同时,随着温度的升高,草酸钠的溶解度呈增大的趋势;但当Na2Ok浓度较高时,温度的影响越来越不明显。温度相同时,随着Na2Ok浓度的升高,草酸钠在铝酸钠溶液中的溶解度急剧下降;当Na2Ok浓度达到200 g/L以上时,继续提高浓度对草酸钠溶解度的影响越来越小。
3 计算模型的扩展及应用
3.1 碳酸钠、硫酸钠对铝酸钠溶液中草酸钠溶解度的影响
工业铝酸钠溶液成分较为复杂,溶液中除主体的Na+、Al(OH)4-、OH-离子之外,还含有一定量的 和
和 。根据Bromley方程,溶液中每一种离子的存在都会对草酸钠的平均活度因子造成影响,继而影响草酸钠在铝酸钠溶液中的溶解度。在复杂的铝酸钠溶液体系中,由已知的
。根据Bromley方程,溶液中每一种离子的存在都会对草酸钠的平均活度因子造成影响,继而影响草酸钠在铝酸钠溶液中的溶解度。在复杂的铝酸钠溶液体系中,由已知的 = 0.0001和
= 0.0001和 = -0.0166,计算出碳酸钠、硫酸钠存在的情况下草酸钠在铝酸钠溶液中的解度,其结果如图3所示。
= -0.0166,计算出碳酸钠、硫酸钠存在的情况下草酸钠在铝酸钠溶液中的解度,其结果如图3所示。
由图3(a)可知,在计算范围内,Na2Ok浓度较低时,溶液中碳酸钠浓度(以Na2OC计)升高,草酸钠的溶解度逐渐下降;当在Na2Ok浓度较高时,草酸钠的溶解度随着碳酸钠浓度的升高而有上升的趋势,但变化很小。由图3(b)可知,硫酸钠的加入对草酸钠溶解度的影响很小,随着铝酸钠溶液中硫酸钠浓度(以Na2Os计)的逐渐升高,草酸钠溶解度变化并不显著。综上所述,可以认为在工业生产过程中,铝酸钠溶液中碳酸钠和硫酸钠的存在对草酸钠的溶解度几乎无影响。

图3 碳酸钠和硫酸钠浓度对铝酸钠溶液中草酸钠溶解度的影响(αk=3,T=353 K)
Fig. 3 Effects of sodium carbonate concentration(a) and sodium sulfate(b) on solubility of sodium oxalate in sodium aluminate solution (αk=3, T=353 K)
3.2 温度对铝酸钠溶液中草酸钠溶解度的影响
温度对草酸钠的溶解度具有重要的影响。应用铝酸钠溶液中草酸钠溶解度的计算模型,本文作者分别研究不同苛性碱浓度时温度对草酸钠溶解度的影响,其结果如图4所示。
由图4可知,碱浓度相同时,随着温度的升高,铝酸钠溶液中草酸钠的溶解度逐渐升高,但随着温度的升高,温度对草酸钠溶解度的影响越来越小。这一结论对采用蒸发结晶法脱除草酸钠具有指导意义。结晶温度对草酸钠的脱除具有重要的作用,结晶温度的降低有助于溶液中草酸钠的结晶析出,但是过低温度也会使得溶液粘度升高,使得析出的草酸钠难以沉降,陈文汨等[5]的研究指出,结晶温度控制为40 ℃最为适宜。

图4 温度对铝酸钠溶液中草酸钠溶解度的影响(αk=3)
Fig. 4 Effect of temperature on solubility of sodium oxalate in different sodium aluminate solutions (αk=3)
3.3 苛性比对铝酸钠溶液中草酸钠溶解度的影响
拜耳法氧化铝生产过程中,铝酸钠溶液的苛性比不断变化,从而影响草酸钠的溶解度。利用草酸钠的溶解度计算模型,本文作者继续计算了苛性比对铝酸钠溶液中草酸钠溶解度的影响,其结果如图5所示。
图5表明,当Al2O3或Na2Ok浓度相同时,随着苛性比值的增大,草酸钠的溶解度逐渐降低。Na2Ok浓度升高以及Al2O3浓度降低均有助于降低铝酸钠溶液中草酸钠的溶解度。DONALD等[20]对草酸钠在氢氧化钠溶液中的溶解度进行研究,并通过向铝酸钠溶液中逐步加碱的方式使得草酸钠过饱和析出,获取较好的效果。
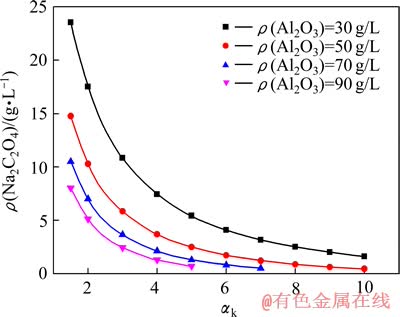
图5 苛性比对铝酸钠溶液中草酸钠溶解度的影响(T=353 K)
Fig. 5 Effect of caustic molar ratio on solubility of sodium oxalate in sodium aluminate solution (T=353 K)
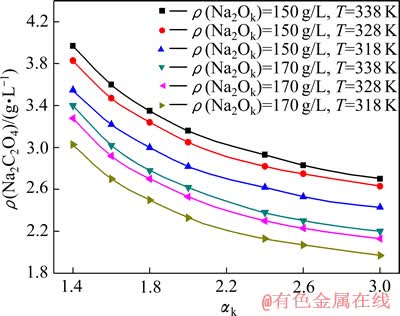
图6 晶种分解过程中草酸钠溶解度的变化
Fig. 6 Variation of solubility of sodium oxalate in seeding precipitation process
利用苛性比对铝酸钠溶液中草酸钠溶解度的影响可以解释草酸钠在晶种分解过程中的结晶析出行为,其结果如图6所示。在晶种分解过程中,铝酸钠溶液的温度逐渐降低,苛性比逐渐升高,两种因素均会导致草酸钠的溶解度降低,最终使其结晶析出。
4 结论
1) 利用草酸钠在水溶液及氢氧化钠溶液中的溶解度数据,计算得到草酸钠的Bromley参数为-0.045,以此为基础建立了铝酸钠溶液体系中草酸钠溶解度计算模型。
2) 纯铝酸钠溶液体系中,草酸钠溶解度的计算数据与文献数据的吻合度较高,说明该模型可以很好地预测铝酸钠溶液体系中草酸钠的溶解度。
3) 利用模型模拟计算拜耳法氧化铝生产过程中草酸钠平衡浓度的变化规律,结果表明:碱浓度越高、温度越低、苛性比越高,铝酸钠溶液中草酸钠平衡浓度越低;溶液中碳酸钠、硫酸钠的存在对草酸钠溶解度的影响很小。
REFERENCES
[1] 李旺兴. 氧化铝生产理论与工艺[M]. 长沙: 中南大学出版社, 2011: 234-237.
LI Wang-xing. Theory and process of alumina product[M]. Changsha: Central South University Press, 2011: 234-237.
[2] MYONG J K, SEONG O L. Overview of the behaviour of sodium oxalate in Bayer liquor and its effect of the process[J]. Light Metals, 2003(5): 19-24.
[3] 尹周澜, 敬叶灵, 陈启元, 张艾民. 聚合物对铝酸钠溶液种分过程的影响[J]. 中国有色金属学报, 2007, 17(6): 1002-1007.
YIN Zhou-lan, JING Ye-ling, CHEN Qi-yuan, ZHANG Ai-min. Effect of polymers on seed precipitation of sodium aluminate solution[J]. The Chinese Journal of Nonferrous Metals, 2007, 17(6): 1002-1007.
[4] 陈文汨, 付云枫. 一种处理从工业铝酸钠溶液中结晶出来的草酸钠的方法: 中国, 201110285220.X[P]. 2011-09-23.
CHEN Wen-mi, FU Yun-feng. Method of sodium oxalate crystallization from sodium aluminate solution: China, 201110285220.X[P]. 2011-09-23.
[5] 陈文汨, 郭金权, 陈学刚. 结晶法去除工业铝酸钠溶液中草酸钠的研究[J]. 轻金属, 2008(11): 12-15.
CHEN Wen-mi, GUO Jin-quan, CHEN Xue-gang. Study of removal of sodium oxalate from sodium aluminate by crystallization process[J]. Light Metals, 2008(11): 12-15.
[6] GERVAIS S, JACQUES E, LAROCQUE G F. Organic control technologies in Bayer process[J]. Light Metals, 2004, 17(7): 109-114.
[7] TAYLOR M, HARRIS D J, CHEN H T. Methods and compositions for the removal of impurities and water from the Bayer process: United States, US 20090169447 A1[P]. 2009-07-02.
[8] 皮溅清, 赵 瑜, 刘桂华, 王永林. 一种铝酸钠溶液石灰苛化除草酸钠的方法: 中国, 201310309531.4[P]. 2013-07-23.
PI Jian-qing, ZHAO Yu, LIU Gui-hua, WANG Yong-lin. Method of sodium oxalate removal from sodium aluminate solution by causticization using lime: China, 201310309531.4[P]. 2013-07-23.
[9] 张佰永, 齐东华. 一种铝酸钠溶液中草酸盐的去除系统及方法: 中国, 200810011314.6[P]. 2008-05-08.
ZHANG Bai-yong, QI Dong-hua. A systerm and method of removal of sodium oxalate from sodium aluminate solution: China, 200810011314.6[P]. 2008-05-08.
[10] THE P J, BUSH J F. Solubility of sodium oxalate in Bayer liquor and a method of Control[J]. Light Metals, 1997, 35(4): 5-10.
[11] POWER G, LOH J. Organic compounds in the processing of lateritic bauxites to alumina. Part 1: Origins and chemistry of organics in the Bayer process[J]. Hydrometallurgy, 2010, 105(1/2): 11-15.
[12] GILBERT B, PHILIPPONNEAU. Physical chemistry models of oxalate and Gibbsite solubilities in Bayer solutions[J]. Light Metals, 1991, 15(8): 97-102.
[13] REYNOLDS J G. Application of mixture models to solubility calculations, using sodium oxalate as an example[J]. Separation Science and Technology, 2008, 43(9/10): 2872-2886.
[14] 覃东棉, 刘桂华, 李小斌, 彭志宏, 周秋生. Debye-Hückel理论的研究进展[J]. 材料导报: 综述篇, 2010, 24(7): 107-112.
QIN Dong-mian, LIU Gui-hua, LI Xiao-bin, PENG Zhi-hong, ZHOU Qiu-sheng. Research progress of Debye-Hückel theory[J]. Material Herald: Review articles, 2010, 24(7): 107-112.
[15] BROMLEY L A. Thermodynamic properties of strong electrolytes in aqueous solutions[J]. American Institute of Chemical Engineers, 2004, 19(2): 313-320.
[16] BORGE G, CASTANO R, CARRIL M P. Development of a modified Bromley's methodology (MBM) for the estimation of ionic media effects on solution equilibria: Part 1. Calculation of the interaction parameters in the molar and molal scales at 25°C[J]. Fluid Phase Equilibria, 1996, 121(1): 85-98.
[17] RAPOSO J C, SANZ J, BORGE G. Development of a modified Bromley's methodology for the estimation of ionic media effects on solution equilibria: Part 3. Application to the construction of thermodynamic models[J]. Fluid Phase Equilibria, 1999, 155(1): 1-19.
[18] RAPOSO J C, ZULOAGA O, OLAZABAL M A. Development of a modified Bromley methodology for the estimation of ionic media effects on solution equilibria: Part 5. The chemical model of boric acid in aqueous solution at 25 °C and comparison with arsenious acid[J]. Fluid Phase Equilibria, 2003, 207(1): 81-95.
[19] BATELLA M, ALEXANDER A, ELI K. The molar enthalpies of solution and solubilities of ammonium, sodium and potassium oxalates in water[J]. Chem Thermodynamics, 2004 (36): 41-47.
[20] DONALD J. Purification of Bayer process liquors: United States, US 4443416[P]. 1984-04-17.
[21] 彭小奇, 宋国辉, 宋彦坡. NaOH-NaAl(OH)4-Na2CO3-H2O体系活度因子的计算模型[J]. 中国有色金属学报, 2009, 19(7): 1332-1337.
PENG Xiao-qi, SONG Guo-hui, SONG Yan-po. Calculation model of activity factor of NaOH-NaAl(OH)4-Na2CO3-H2O system[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(7): 1332-1337.
(编辑 李艳红)
收稿日期:2014-03-05;修订日期:2014-07-10
通信作者:齐天贵,讲师,博士;电话:0731-88877830;E-mail: qtg_csu@163.com