Supermolecular template route to fabrication of well crystallized hollow antimony microspheres
来源期刊:中国有色金属学报(英文版)2006年第2期
论文作者:谷俐 陈树大 韦晓燕
文章页码:346 - 350
Key words:supermolecular template route; poly(ethylene glyco); oleic acid; antimony; crystallinity; hollow spheres
Abstract: Hollow spheres of elemental antimony (Sb) with good crystallinity, high contrast and thin wall were prepared in the solutions of poly(ethylene glycol) (PEG) and oleic acid(OA) associations at the refluxed temperature. The complexes of Sb3+ with tartaric acid were used as precursors, which can avoid the hydrolysis of SbCl
基金信息:the Science Foundation of Department of Education of Zhejiang Province, China
the Science Foundation of Jiaxing Science and Technology Bureau, China

GU Li(谷 俐), CHEN Shu-da(陈树大), WEI Xiao-yan(韦晓燕)
College of Biology and Chemical Engineering, Jiaxing University, Jiaxing 314001, China
Received 20 June 2005; accepted 10 January 2006
Abstract: Hollow spheres of elemental antimony (Sb) with good crystallinity, high contrast and thin wall were prepared in the solutions of poly(ethylene glycol) (PEG) and oleic acid(OA) associations at the refluxed temperature. The complexes of Sb3+ with tartaric acid were used as precursors, which can avoid the hydrolysis of SbCl3 and the resulting impurity of products. The average diameter and thickness of the as-prepared hollow sphere are about 300 nm and less than 20 nm, respectively. The formation of hollow spheres depends on the template function of PEG and OA associations, which can be confirmed through the theoretical analysis and results of control experiments. The specific surface area reaches 34.669 m2/g.
Key words: supermolecular template route; poly(ethylene glyco); oleic acid; antimony; crystallinity; hollow spheres
1 Introduction The preparation of hollow spheres in the nanometer to micrometer scale is of particular interest due to their specific structure and wide applications in artificial cells[1], light-mass structured materials[2,3], catalysts[4], controlled release capsules of drugs, cosmetics and dyes[1, 5], and so on. These characters origin from the unique properties of hollow spheres, such as the low density, mechanical stability, large specific area and surface permeability. Up to date, a large number of substances, including metallic elements[4, 6-8], metal chalcogenides[9, 10], organic-inorganic hybrids and organic polymer[11,12] have been fabricated into hollow spheres through various approaches. The conventional method for preparing inorganic hollow spheres is through the layer-by-layer(LbL) coating process[13], which usually needs a sacrificial template (e.g. polymer latex particles or silica spheres). In addition, microemulsions[14], block copolymers[15], liquid- droplets[9], vesicles[10, 16] and resin spheres[17] can also be used as templates for the formation of hollow spheres, including inorganic and organic substances. Although many methods have been developed during the last years, the progress in the preparation of hollow spheres of metallic and semi-metallic elements is very little except the reports on those whose potentials are lower than that of hydrogen (e.g. copper, silver and gold)[6-8]. But hollow structures of metal elements are important catalysts, sensors and substrates in surface-enhanced Raman scattering(SERS) and optical materials[7]. The difficulties for obtaining their hollow structures are partly attributed to their high potential, instability at atmosphere or strong hydrolysis tendency of their precursors (such as SbCl3 and BiCl3). The above-mentioned difficulties prevent the homogeneous reduction of metallic ions in moderate conditions, whereas severe experimental conditions (e.g. high temperature and strong acidity/alkalinity) will cause the collision of the assembled structures of some applied templates. So, the most important things for obtaining the hollow spheres of such metallic elements are to find appropriate precursors and stable templates. Recently, the associations of polymer and surfactant are found to be a promising template for the production of nanomaterials with specific morphologies. Water-soluble polymers interact strongly with surfactants in aqueous solutions. The surfactants associating with the polymer chains form micellar aggregates at a critical association concentration (cac), which is often considerably lower than the critical micellar concentration (cmc) of the surfactant in pure water[18-21]. Since the synthetic effect and some complicated patterns can be contributed by the supermolecular associations of water-soluble polymers and anionic/cationic surfactants, various nanostructures, including hollow spheres, nanowires, nanorod junctions and monodispersed quantum dots can be obtained facilely and rapidly in some researches[7, 18-24]. Various methods, including the protection by inert gas and coordination, can be applied in the reaction involving some unstable precursors. As for some precursors with a strong hydrolysis tendency in the aqueous solution, they can be turned into water-soluble ions through the formation of complexes with appropriate ligands. For example, the precipitation of antimony oxychloride (SbOCl) can be avoided if SbCl3 is dissolved in the aqueous solution of tartaric acid. In the present paper we report an improved aqueous-chemistry process, which allows to synthesize submicrometer-scaled hollow spheres of antimony with the Sb3+ complexes and associations of poly(ethylene glycol) (PEG) and oleic acid(OA) as precursors and templates, respectively. 2 Experimental
2.1 Preparation of hollow antimony microspheres
Antimony chloride(SbCl3), tartaric acid(C4H6O6), PEG(Mn=10 000) and oleic acid(OA) were all used without further treatment. A 0.01 mol/L aqueous solution of potassium borohydride (KBH4) was obtained by dissolving 0.026 g KBH4 in 50 mL deionized water. A typical experimental process was as follows. 1 g PEG and 0.5 mL OA were dissolved in 100 mL H2O in a conical beaker and this solution was sonicated for 20 min to ensure OA be dispersed homogeneously. Then, 0.002 mol SbCl3 was put into the above solution and white antimony oxychloride (SbOCl) precipitated quickly, which could be resolved by the addition of 2 g C4H6O6. Afterward, the solution was heated and refluxed at 100 ℃ for 30 min and 0.01 mol/L KBH4 solution was dropped into the conical beaker. The solution turned into gray gradually, indicating the formation of Sb element. After the titration, the solution was kept reflux for 3 h. The final gray precipitation was separated from the solution by centrifugation at a speed of 3 000 r/min, and then washed with distilled water and n-hexane repetitively to remove the remained PEG and OA, and then dried in vacuum at 40 ℃ for 5 h.
2.2 Characterization of hollow antimony micro- spheres
The X-ray powder diffraction(XRD) pattern of the as-prepared product was recorded on a Rigaku D/max gA rotation anode diffractometer using Ni-filtered Cu Ka radiation. Transmission electron microscopy(TEM) and selected area electronic diffraction(SAED) examinations were carried out on a Hitachi Model H-800 microscope, using an accelerating voltage of 200 kV. The field emission scanning electron microscopy (FE-SEM) images were taken on a JEOL JSM–6700F microscope. The specific surface area of the product was measured by BET adsorption (Micrometric Instrument Co. ASAP 2000 series) in a nitrogen atmosphere.
3 Results and discussion
3.1 Morphology and structure of synthesized Sb hollow spheres
X-ray diffraction was used to characterize the phase and the crystallographic structure of the as-prepared product. The diffraction pattern of product is shown in Fig.1. All the reflections in the pattern can be indexed as rhombohedral Sb with the lattice parameters a=0.431 nm and c=1.118 nm, which are close to the reported data (Joint Committee on Powder Diffraction Standards, JCPDS files No. 35-732). Meanwhile, the XRD pattern shows that the product is well crystallized and no impurities such as Sb(OH)Cl and/or SbOCl are detected. The good crystallinity is attributed to the high reaction temperature (about 100 ℃). The high purity of the as-prepared products is because that the precipitation of Sb(OH)Cl can be avoided when SbCl3 is treated with tartaric acid. The combination of Sb3+ complexes with the reducing agent of KBH4 leads to the conversion of Sb3+ complexes to Sb without other side reactions.
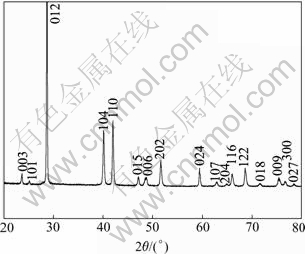
Fig.1 XRD pattern of product
Fig.2(a) shows that Sb nanocrystals are hollow spheres in structure, which can be learned from the strong contrast between the dark edge and pale center. The resolved average diameter for hollow Sb spheres is about 300 nm. The TEM and FE-SEM observations of broken hollow spheres, as shown in Figs.2(b) and (c), further confirm the hollow nature of the product. Fig.2(d) shows the corresponding selected area electronic diffraction(SAED) pattern of hollow spheres, which indicates the good crystallinity of the products, consist with the result of XRD.
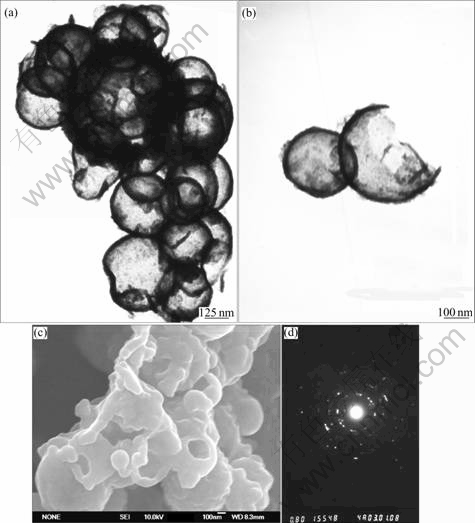
Fig.2 TEM images of hollow antimony microspheres(a) and broken hollow antimony microspheres(b), FE-SEM image of hollow antimony microspheres(c), and SAED pattern of hollow antimony microspheres(d)
Although some fine particles are adsorbed on the surface of hollow spheres, they can be removed easily through the repetitive centrifugal separation. First, the hollow spheres were dispersed in absolute alcohol and the obtained suspension was centrifugalized at the speed of 5 000 r/min. Then, the supernatant liquid was discarded and the collected precipitation was re-dispersed in absolute alcohol for further centrifugation. To obtain high quality hollow spheres, such processes should be repeated at least three times. From the TEM image one can see that there are a smoother wall and a higher contrast between the pale center and dark edge after such a process (Fig.3). Moreover, the thickness of the edge is less than 20 nm, which is much thinner than that obtained by the LbL process[13]. Hollow antimony microspheres were also characterized by FE-SEM, and a typical image is shown in Fig.3.
3.2 Formation mechanism of hollow antimony spheres
The formation of hollow spheres is attributed to the template function of PEG-OA associations, which is schematically described in Fig.4. When oil-soluble oleic acid is added into the transparent aqueous solution of PEG, the color of the solution turns into cream white after 15 min ultrasonic dispersion, which reveals that the interactions between PEG chains and OA molecules occur and PEG-OA associations are formed[22]. Because of the presence of the hydrophobic OA, PEG-OA associations exist in the form of oil-in-water droplets in the solution. Such oil-in-water droplets should act as templates for the development of hollow sphere structures. Additionally, since the precursors for the formation of hollow spheres are negative [SbO(C4H4O6)]- rather than positive Sb3+ (reaction 1 and 2), there is a strong electrostatic repulsion arising from the interactions of anionic OA and [SbO(C4H4O6)]- ions. And the electrostatic repulsion leaves [SbO(C4H4O6)]- ions to be dispersed in the outer of the oil-in-water droplets. The reduction of them by KBH4 allows Sb nanocrystals to nucleate and grow near the periphery of droplets and form hollow structure finally. Because the electrostatic repulsion between [SbO(C4H4O6)]- ions and anionic OA prevents the former from entering the inner of the droplets and their reduction is limited in the periphery of the droplets, the quality of the obtained hollow spheres is obviously higher than that obtained through an electrostatic attraction mechanism[7,10]. Furthermore, the results of controlled experiment provide experimental evidence and further confirm the template function of PEG-OA associations. When the templates are replaced by PEG or OA, respectively, the morphologies of the products are irregular nanoparticles, which are totally different from the hollow spheres obtained in the presence of PEG-OA associations (Figs.5(a) and (b)). They demonstrate that the formation of hollow spheres needs the synergistic effect of PEG and OA.
Hydrolysis of SbCl3:
SbCl3+H2O→HCl+Sb(OH)Cl (insoluble) (1)
Complexing with C4H6O6:
Sb(OH)Cl+C4H6O6→[(SbO)C4H4O6]Cl (soluble) (2)
Reduction by KBH4:
[(SbO)C4H4O6]Cl![]() Sb (3)
Sb (3)

Fig.3 TEM image of hollow antimony microspheres after repetitive wash run
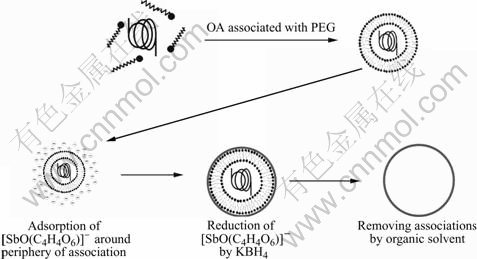
Fig.4 Schematic description of growth process of hollow antimony microspheres
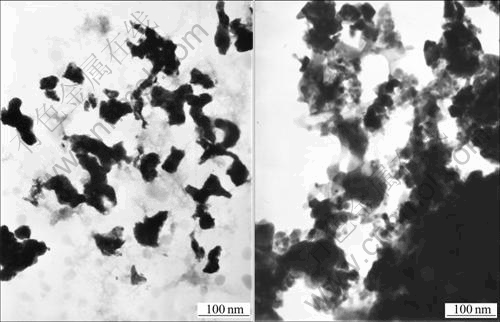
Fig.5 TEM images of Sb prepared with PEG(a) and with OA as controlling agent(b)
3.3 Surface area characterization of hollow antimony microspheres
The specific surface area of hollow spheres is determined to be 34.669 m2/g according to the adsorption and desorption isotherms, which is about two times as that of carbon nanobeads whose diameter is close to that of the as-prepared hollow spheres[25]. The larger specific surface area of the hollow spheres in the present study should be attributed to the existence of large amounts of broken spheres, which lead to the increase of the specific surface area.
4 Conclusions
The ultra-thin and well crystallized antimony hollow spheres were prepared in the aqueous solution at a refluxed temperature. The associations of PEG and OA were the templates for the nucleation and growth of hollow spheres and the Sb3+ complexes were the precursors. Without the synergistic effect of PEG and OA, the product with irregular shape would be obtained. The specific surface area of hollow spheres is two times as that of carbon nanobeads because there are large amounts of broken spheres in the products.
References
[1] Li W J, Sha X X, Dong W J, Wang Z C. Synthesis of stable hollow silica microspheres with mesoporous shell in nonionic W/O emulsion[J]. Chem Commun, 2002, 200: 2434-2435.
[2] Walsh D, Mann S. Fabrication of hollow porous shells of calcium carbonate from self-organizing media[J]. Nature, 1995, 377(28): 320-324.
[3] Caruso F. Hollow capsule processing through colloidal templating and self-assembly[J]. Chem Eur J, 2000, 6(3): 413-419.
[4] Kim S W, Kim M, Lee W Y, HYZON T. Fabrication of hollow palladium spheres and their successful application to the recyclable heterogeneous catalyst for Suzuki coupling reactions[J]. J Am Chem Soc, 2002, 124(26): 7642-7643.
[5] Muthusamy E, Walsh D, Mann S. Morphosynthesis of organoclay microspheres with sponge-like or hollow interiors[J]. Adv Mater, 2002, 14(13): 969-972.
[6] Kawahashi N, Shiho H. Copper and copper compounds as coatings on polystyrene particles and as hollow spheres[J]. J Mater Chem, 2000, 10: 2294-2297.
[7] Zhang D B, Qi L M, Ma J M, CHENG H. Synthesis of submicrometer-sized hollow silver spheres in mixed polymer-surfactant solutions[J]. Adv Mater, 2002, 14(20): 1499-1502.
[8] Caruso F, Spasova M, Saigueirino-Maceira V, LIZ-MARZA L M. Multilayer assemblies of silica-encapsulated gold nanoparticles on decomposable colloid templates[J]. Adv Mater, 2001, 13(14): 1090-1094.
[9] Huang J X, Xie Y, Li B, QIAN Y T, ZHANG S H. In-situ source-template-interface reaction route to semiconductor CdS submicrometer hollow spheres[J]. Adv Mater, 2000, 12(11): 808- 811.
[10] Zheng X W, Xie Y, Zhu L Y, YAN A H. Formation of vesicle-templated CdSe hollow spheres in an ultrasound-induced anionic surfactant solution[J]. Ultrasonics Sonochemistry, 2002, 9(6): 311-316.
[11] Caruso F, Caruso R A, Mohwald H. Production of hollow microspheres from nanostructured composite particles[J]. Chem Mater, 1999, 11(11): 3309-3314.
[12] Wei Z X, Wang M X. Hollow microspheres of polyaniline synthesized with an aniline emulsion template[J]. Adv Mater, 2002, 14(18): 1314-1317.
[13] Caruso R A, Susha A, Caruso F. Multilayered titania, silica, and laponite nanoparticle coatings on polystyrene colloidal templates and resulting inorganic hollow spheres[J]. Chem Mater, 2001, 13(2): 400-409.
[14] Walsh D, Lebeau B, Mann S. Morphosynthesis of calcium carbonate(vaterite) microsponges[J]. Adv Mater, 1999, 11(4): 324-328.
[15] Liu T B, Xie Y, Chu B. Use of block copolymer micelles on formation of hollow MoO3 nanospheres[J]. Langmuir, 2000, 16(23): 9015-9022.
[16] Hubert D H W, Jung M, Frederik P M, BOMANS P H H, MEULDIJK J, GERMAN A L. Vesicle-directed growth of silica[J]. Adv Mater, 2000, 12(17): 1286-1290.
[17] Bourlinos a b, Karakassides m a, Petridis d. Synthesis and characterization of hollow clay microspheres through a resin template approach[J]. Chem Commun, 2001, 16: 1518.
[18] Fox G J, Bloor D M, Holzwarth J F, WYN-JONES E. Use of isothermal titration microcalorimetry to monitor the adsorption/desorption processes of sodium dodecyl sulfate with neutral polymers[J]. Langmuir, 1998, 14(5): 1026-1030.
[19] Leontidis E, Kyprianidou-Leodidou T, Caseri W, ROBYR P, KRUMEICH F, KYRIACOU K C. From colloidal aggregates to layered nanosized structures in polymer-surfactant systems(Ⅰ): Basic phenomena[J]. J Phys Chem B, 2001, 105(19): 4133-4144.
[20] Nizri G, Magdassi S, Schmidt J, GOHEN Y, TALMON Y. Microstructural characterization of micro- and nanoparticles formed by polymer-surfactant interactions[J]. Langmuir, 2004, 20(11): 4380-4385.
[21] Cao X B, Yu F, Li L Y, YAO Z Y, XIE Y. Copper nanorod junctions templated by a novel polymer–surfactant aggregate[J]. J Crystal Growth, 2003, 254(1): 164-168.
[22] Huang S P, Zhou K C, Liu Y, HUANG B Y. Controlled crystallization of hydroxyapatite under hexadecylamine self-assembled monolayer[J]. Trans Nonferrous Met Soc China, 2003, 13(3): 595-599.
[23] Zhang C F, Zhan J, Wu J H. Preparation and characterization of fibrous NiO particles by thermal decomposition of nickelous complex precursors[J]. Trans Nonferrous Met Soc China, 2004, 14(4): 713-717.
[24] Qi L M, Li J, Ma J M. Biomimetic morphogenesis of calcium carbonate in mixed solutions of surfactants and double-hydrophilic block copolymers[J]. Adv Mater, 2002, 14(4): 300-303.
[25] Sharon M, Mukhopadhyay K, Yase K, IJIMA S, ANDO Y, ZHAO X L. Spongy carbon nanobeads—A new material[J]. Carbon, 1998, 36(5): 507-511.
Foundation item: Project(20030756) supported by the Science Foundation of Department of Education of Zhejiang Province, China; Project (20041007) supported by the Science Foundation of Jiaxing Science and Technology Bureau, China
Corresponding author: GU Li; Tel/Fax: +86-573-3646246; E-mail: guli@mail.zjxu.edu.cn

