
Leaching vanadium by high concentration sulfuric acid from stone coal
CHEN Xiang-yang(陈向阳)1, 2, LAN Xin-zhe(兰新哲)1, 2, ZHANG Qiu-li(张秋利)1,
MA Hong-zhou(马红周)1, ZHOU Jun(周 军)1
1. School of Metallurgical Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, China
2. Research Center of Metallurgical Engineering and Technology of Shaanxi Province, Xi’an 710055, China
Received 6 July 2009; accepted 30 December 2009
____________________________________________________________________________________________
Abstract: An effectively new technology of extracting vanadium from stone coals by high concentration sulfuric acid was researched. The effect of the concentration of sulfuric acid, leaching temperature, leaching time and helper leaching agent on the extraction of vanadium was explored. The results show that the optimal conditions of extraction are as follows: the concentration of sulfuric acid is 6 mol/L, the ratio of liquid to solid is 3?1; the temperature is 90 ℃; the leaching time is 3-5 h, the diameter of the ore particle is less than 180 μm, and the concentration of helper leaching agent R is 6%. Under these conditions, the extraction of vanadium can reach 95.86%.
Key words: stone coal; vanadium; leaching; sulfate solution
____________________________________________________________________________________________
1 Introduction
Vanadium is an important product that is used almost in ferrous and non-ferrous alloys due to its high physical properties such as tensile strength, hardness, and fatigue resistance[1-2]. In addition, there is significant potential use for vanadium in vanadium redox battery. Vanadium is never found in its pure state but it occurs in combination with various minerals[3-9]. The stone coal ore is one of the important vanadium sources in China. The brief flow of the classical process leaching vanadium from stone coal includes chloridizing roasting, water leaching, deposition, alkali melting and thermal decomposition[9-12]. This process is complicated with low recovery of vanadium[11-12]. Moreover, it causes serious environmental pollution by poisonous gases and waste water from the process[9]. In recent years, many researches have been focused on leaching vanadium from stone coal by low concentration sulfuric acid[11-13]. However, this process has weak intensity of reaction and low recovery of vanadium[14-17]. So, it is necessary to research a high-recovery technology of extracting vanadium from stone coal.
The aim of this work is to extract vanadium from stone coal using high concentration sulfuric acid. The effect of the concentration of sulfuric acid, leaching temperature, leaching time and helper leaching agent on the extraction of vanadium was researched.
2 Experimental
2.1 Materials
The vanadium ore used in this study was obtained from a certain place in Shaanxi Province, China. The main chemical compositions in vanadium ore (Table 1) show that the content of vanadium (V2O5) is 1.090 0%.
Table 1 Chemical compositions of vanadium ore (mass fraction, %)

2.2 Methods
The leaching experiments were performed in a 300 mL three-necked flask heated in a water bath kept constant within ±1.0 ℃ and connected to a mechanical agitator, equipped with twin-bladed impeller and shaft coated by Teflon. For minimizing aqueous loss, a reflux condenser was applied. When the desired temperature was reached, 40 g vanadium ore (the diameter of the ore particle is less than 180 μm) was added to sulfuric acid solution stirred at 900 r/min. After a scheduled duration, the leach liquor was separated from the residue by vacuum filtration. The experiments were conducted under different conditions of sulfuric acid concentration, leaching temperature, leaching time and helper leaching agent.
3 Results and discussion
3.1 Effect of sulfuric acid concentration
The effect of sulfuric acid concentration in 1-10 mol/L was studied for 2 h at 80 ℃ with the ratio of solid mass to liquid volume 1?3 g/mL. The results is shown in Fig.1. It can be seen from Fig.1 that with the increase of sulfuric acid concentration, the extraction of vanadium increases. This phenomenon is attributed to the fact that higher concentration facilitates the replacement of vanadium atoms, because H+ has sufficient energy to damage the lattice energy between the illite and vanadium mica. The extractive reaction is as follows:
2V2O3+8H++O2=VO2++H2O (1)

Fig.1 Effect of sulfuric acid concentration on extraction of vanadium
When the acid concentration is 6 mol/L, 79.88% vanadium is extracted. Further increasing sulfuric acid concentration has no remarkable variation in the extraction of vanadium, but more Al and Fe are leached. Therefore, 6 mol/L H2SO4 was chosen for the subsequent experiments[5-6].
3.2 Effect of leaching temperature
The effect of temperature on vanadium extraction (leaching time 2 h) was examined in 75-95 ℃ as shown in Fig.2. It can be seen from Fig.2 that the higher temperature can increase the speed of molecular motion and the collisions between the vanadium atoms and H+. That temperature has a profound effect on increasing vanadium extraction, indicating that the dissolution process is controlled by a chemical reaction. A maximum extraction of 85.86% is achieved at 95 ℃, but 85.06% is extracted at 90 ℃, and this temperature is chosen to be optimum for saving energy[5].

Fig.2 Effect of leaching temperature on extraction of vanadium
3.3 Effect of helper leaching agent
Fig.3 shows the effect of the helper leaching agent on the extraction of vanadium. It is clearly found that the extraction rate of vanadium with the helper leaching agent R is the highest, suggesting that the helper leaching agent R significantly improves the extraction rate of vanadium. The extraction rate of the vanadium with the helper leaching agent MnO2 and H2O2 is lower than that with the helper leaching agent R. The reason is that the helper leaching agent MnO2 consumes a part of sulfuric acid solution and takes Mn2+ into the reaction system, which prohibits the extractive reaction. The helper leaching agent H2O2 is easily decomposed at higher temperature. On the contrary, the helper leaching agent FeSO4 decreases the extraction rate of the vanadium. This result is attributed to the fact that Fe2+ is the reducing ion which reverses the direction of extractive reaction.

Fig.3 Effect of different helper leaching agents on extraction of vanadium
Fig.4 shows that the effect of helper leaching agent R on the extraction of vanadium under the condition of the concentration of H2SO4 6 mol/L, temperature 90 ℃ and leaching time 2 h. It can be found that the extraction rate of vanadium increases with the increase of the concentration of the helper leaching agent R from 1% to 5%. When the concentration of the helper leaching agent R is 5%, 91.86% vanadium is extracted. Further increasing helper leaching agent R concentration gives no remarkable increase in the extraction of vanadium. 6% helper leaching agent R is sufficient to ensure maximum possible extractive reaction.
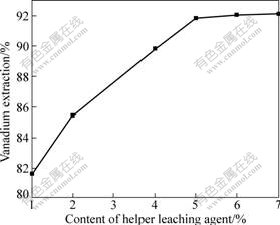
Fig.4 Effect of helper leaching agent R on extraction of vanadium
3.4 Effect of leaching time
The role of leaching time on the extraction of vanadium under the conditions of concentration of H2SO4 6 mol/L, temperature 90 ℃ and leaching time 2 h is shown in Fig.5. It can be seen from Fig.5 that the extraction of vanadium reaches 94.19% rapidly within 3 h, but after that, it increases slowly with 95.96% vanadium extracted after 3 h and no beneficial effect of extended leaching time. The reason is that with the increase of the leaching time, more and more VOSO4 covers on the surface of unreacted particle core. Consequently, the leaching process slows down. Hence, a leaching time of 3-5 h seems to be sufficient to extract appreciable vanadium.
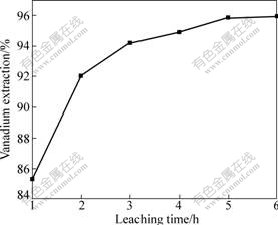
Fig.5 Effect of leaching time on extraction of vanadium
3.5 Effect of experimental condition
Fig.6 shows the effect of experimental condition on the extraction of vanadium. It is clearly found that the extraction rate of vanadium is only 70.13% with the low concentration sulfuric acid, and 90.06% and 95.86% vanadium are extracted under the condition of the high concentration sulfuric acid without and with 6% helper agent R, respectively, indicating that the high concentration sulfuric acid with 6% helper agent R is the optimal condition.
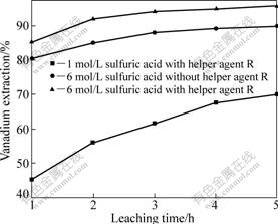
Fig.6 Extraction of vanadium under different conditions
4 Conclusions
1) The extraction rates of vanadium from stone coal ore are 70.13%, 90.06% and 95.86% under the condition of the low concentration sulfuric acid 1 mol/L, high concentration sulfuric acid 6 mol/L without helper leaching agent and high concentration sulfuric acid 6 mol/L with 6% helper leaching agent R, respectively. The helper leaching agent R can significantly increase the extraction rate of vanadium.
2) The optimal conditions of extraction are as follows: The sulfuric acid concentration is 6 mol/L, the ratio of liquid to solid is 3?1 mL/g, the temperature is 90 ℃, the leaching time is 3-5 h, the diameter of the ore particle is less than 180 μm, and the concentration of helper leaching agent R is 6%. Under these conditions, the extraction of vanadium can reach 95.86%.
References
[1] Moskalyk R R, Alfantazi A M. Processing of vanadium: A review [J]. Minerals Engineering, 2003, 16(9): 793-805.
[2] Navarro R, Guzman J, Saucedo I, Revilla J, Guibal E. Vanadium recovery from oil fly ash by leaching, precipitation and solvent extraction processes [J]. Waste Management, 2007, 27(3): 425-438.
[3] Zeng L, Li Q g, Xiao L s. Extraction of vanadium from the leach solution of stone coal using ion exchange resin [J]. Hydrometallurgy, 2009, 97: 194-197.
[4] LI qing-gang, XIAO Lian-sheng, ZHANG Gui-qing, ZHANG Qi-xiu. Process and practice of ammonium molybdate production from Ni-Mo ore by hydrometallurgy [J]. Chinese Journal of Rare Metals, 2007, 31(s1): 64-69. (in Chinese)
[5] Shao Y h, Feng Q m, Chen Y, Ou L m, Zhang G f, Lu Y p. Studies on recovery of vanadium from desilication residue obtained from processing of a spent catalyst [J]. Hydrometallurgy, 2009, 96: 166-170.
[6] STAS J, DAHDOUH A, Al-chayah O. Recovery of vanadium, nickel and molybdenum from fly ash of heavy oil-fired electrical power station [J]. Periodica Polytechnica:Chemical Engineering, 2007, 51(2): 67-70.
[7] TAKAYUKI H, ISAO K. Electro-reductive stripping of vanadium in solvent extraction process for separation of vanadium and molybdenum using tri-n-octylmethylammonium chloride [J]. Hydrometallurgy, 1993, 33: 73-82.
[8] SHI You-fu, WANG Hai-bei. Separation of molybdenum and vanadium from spent catalysts [J]. China Molybdenum Industry, 2004, 28(2): 39-41. (in Chinese)
[9] Dai Wen-can, Zhu Qi-jin, Chen Qing-bang, Liu Ru-yi, Sun Shui-yu. The study of comprehensive utilisation of new processes on the extraction of vanadium from stone coal [J]. Nonferrous Metals: Mineral Processing Section, 2000, 3: 15-17. (in Chinese)
[10] Cai Jin-qiang. The new process of production of vanadium from stone coal [J]. Inorganic Salt Industry, 2001, 33(5): 37-39. (in Chinese)
[11] Mishra D, Kim D J, Ralph D E, Ahn J G, Rhee Y H. Bioleaching of vanadium rich spent refinery catalysts using sulfur oxidizing lithotrophs [J]. Hydrometallurgy, 2007, 88(1/4): 202-209.
[12] Bin Zhi-yong. Progress of the research on extraction of vanadium pentoxide from stone coal and the market of the V2O5 [J]. Hunan Nonferrous Metals, 2006, 22(1): 16-20. (in Chinese)
[13] He D S, Feng Q M, Zhang G F, Ou L M, Lu Y P. An environmental-friendly technology of vanadium extraction from stone coal [J]. Minerals Engineering, 2007, 20(11): 1184-1186.
[14] ZENG Tian-wen, DAI Wen-can, ZHANG Zhi, ZHU Qi-jin. Study on exchanging properties of anion exchange resins for vanadium(V) [J]. Ion Exchange and Adsorption, 2002, 18(5): 453-458. (in Chinese)
[15] Qi Ming-jian. The status and prospects of vanadium leaching from stone coal [J]. Hydrometallurgy of China, 1999, 72(4): 1-10. (in Chinese)
[16] DU Na, CHEN Kun, HU Chuan-qun, CHEN Yan, WANG Yi-yong, WANG Si-hua. Study on extraction of vanadium from vanadium ore by pollution-free technology [J]. Hunan Nonferrous Metals, 2008, 24(5): 17-19. (in Chinese)
[17] Ozawa K, Eguchi M, Sakka Y. Low-temperature preparation of lithium vanadium oxides by solution processing [J]. Journal of the European Ceramic Society, 2004, 24(2): 405-408.
__________________________
Foundation item: Project(2007ZDGC-11) supported by “13115” Science and Technique Innovation Program of Shaanxi Province, China; Project(QN0918) supported by Science Fund for Young Scholars of Xi’an University of Architecture and Technology, China
Corresponding author: CHEN Xiang-yang; Tel: +86-29-82202955; E-mail:chenxiangyang2006@126.com
(Edited by CHEN Can-hua)