体内外实验评估Mg-6Zn合金对肠上皮细胞紧密连接的影响
来源期刊:中国有色金属学报(英文版)2015年第11期
论文作者:于嵩 王啸虎 陈义刚 郑起 张小农 赵常利 张绍翔 阎钧
文章页码:3760 - 3766
关键词:Mg-6Zn合金;肠上皮细胞;紧密连接
Key words:Mg-6Zn alloy; intestinal epithelial cell; tight junction
摘 要:研究Mg-6Zn合金对肠上皮紧密连接的影响。体外实验中,肠上皮细胞(IEC-6)分别在不同浓度(0,20%和40%)合金浸提液中培养1、3和5天。逆转录聚合酶链反应(RT-PCR)法检测结果显示,当IEC-6细胞在40%和20%浓度合金浸提液中培养时,Occludin和ZO-1 mRNA表达水平在各不同的时间点相对于对照组升高。体内实验中,镁合金吻合钉和医用钛吻合钉分别植入兔小肠中1、2和3个星期,采用免疫组织化学染色法检测植入部位周围组织紧密连接相关蛋白的表达。结果显示,植入后第1、2和3星期三个时间点,Mg-6Zn合金组植入部位周围组织中ZO-1、Occludin表达水平明显高于钛钉组和对照组。综上所述,Mg-6Zn合金植入肠道后可促进肠道周围组织紧密连接的再生,其浸提液在一定浓度下可诱导细胞紧密连接相关基因的表达。
Abstract: The effects of biodegradable Mg-6Zn alloy on tight junction of intestinal epithelial cells (IEC-6) were investigated. In the in vitro experiments, the cells were exposed to Mg-6Zn alloy extracts with different concentrations (0, 20% and 40%) for 1, 3 and 5 d. The real-time polymerase chain reaction (PCR) results show that when the cells are treated with 40% and 20% extracts, the expression of Zona Occludens 1 (ZO-1) and Occludin increase as compared with those in the control group. In the in vivo experiments, Mg-6Zn alloy and titanium staples were implanted into rabbits’ intestinal tract for 1, 2 and 3 weeks. By immunohistochemical staining of peri-implant intestinal tissue, increased expression of Occludin and ZO-1 are observed in the Mg-6Zn alloy groups as compared with those in the titanium and control groups. The results show that Mg-6Zn alloy in intestine may promote the regeneration of tight junction, and the extract with a certain concentration can induce the expression of tight junction related genes in IEC-6 cells.
Trans. Nonferrous Met. Soc. China 25(2015) 3760-3766
Song YU1, Xiao-hu WANG1,2, Yi-gang CHEN3, Qi ZHENG1,2, Xiao-nong ZHANG4, Chang-li ZHAO4, Shao-xiang ZHANG5, Jun YAN1
1. Department of General Surgery, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai 200233, China;
2. School of Medicine, Shanghai Jiao Tong University, Shanghai 200025, China;
3. Department of General Surgery, Nanjing Medical University Affiliated Wuxi No. 2 People’s Hospital, Nanjing 214002, China;
4. State Key Laboratory of Metal Matrix Composites, School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China;
5. Suzhou Origin Medical Technology Co. Ltd., Suzhou 215513, China
Received 10 December 2014; accepted 4 August 2015
Abstract: The effects of biodegradable Mg-6Zn alloy on tight junction of intestinal epithelial cells (IEC-6) were investigated. In the in vitro experiments, the cells were exposed to Mg-6Zn alloy extracts with different concentrations (0, 20% and 40%) for 1, 3 and 5 d. The real-time polymerase chain reaction (PCR) results show that when the cells are treated with 40% and 20% extracts, the expression of Zona Occludens 1 (ZO-1) and Occludin increase as compared with those in the control group. In the in vivo experiments, Mg-6Zn alloy and titanium staples were implanted into rabbits’ intestinal tract for 1, 2 and 3 weeks. By immunohistochemical staining of peri-implant intestinal tissue, increased expression of Occludin and ZO-1 are observed in the Mg-6Zn alloy groups as compared with those in the titanium and control groups. The results show that Mg-6Zn alloy in intestine may promote the regeneration of tight junction, and the extract with a certain concentration can induce the expression of tight junction related genes in IEC-6 cells.
Key words: Mg-6Zn alloy; intestinal epithelial cell; tight junction
1 Introduction
At present, due to its good mechanical properties, metallic materials such as stainless steel, Nitinol, titanium and titanium alloys are commonly used as surgical staples and surgical suture material. The use of these materials can shorten the operation time and reduce surgical complications. However, some of these materials show higher elastic modulus as compared with human tissue, producing stress shielding and delay healing. Even, after corrosion, some of them release toxic elements such as nickel and vanadium ions to the surrounding tissue which have the carcinogenic activity [1,2]. Because of the non-degradability, the materials implanted will be long-term retention in human body and cause inconveniences for the patient to take computed tomography examination. What’s more, some of the materials should be removed by invasive second surgery. To avoid the disadvantages of the above materials, various kinds of degradable materials based on the non-toxic elements were designed [3]. A kind of novel binary Mg-6%Zn (mass fraction) (Mg-6Zn) alloy was developed by our research group. In the previous studies, the alloy showed good biocompatibility within the femoral shaft in rabbits in vivo and had a cytotoxicity grade of 0–1 with L-929 and MC3T3-E1 cell in vitro [4-6]. In order to promote the usage of Mg-6Zn alloy in the intestinal tract reconstruction in general surgery, the effects of Mg-6Zn alloy on IEC-6 cells must be evaluated. Though in the previous studies, the influence of Mg-6Zn alloy on the cell cycle and apoptosis of IEC-6 cells has been researched [7,8], the effect of that on other aspects that related with the cell function should also be included.
Tight junctions (TJ) are the most apical intercellular junctions of the lateral membrane in epithelial and endothelial cells, regulating the paracellular passage of ions and molecules through the paracellular pathway and maintain plasma membrane polarity [9,10]. Proper function of epithelia depends on the integrity of tight junctions, and the dysfunction of tight junctions causes many diseases of multiple organs, including the gastrointestinal tract [11,12]. Therefore, the analysis of TJ regulation could lead to an understanding of the use of Mg-6Zn alloy in the general surgery.
The present study aims to investigate the effect of Mg-6Zn alloy on tight junction through in vitro cell experiments and in vivo animal experiments. IEC-6 cells were exposed to Mg-6Zn alloy extracts at different concentrations to evaluate the expressions of tight junction genes such as Occludin and Zona Occludens 1 (ZO-1). Moreover, Mg-6Zn alloy staples and titanium staples were implanted into rabbits’ intestinal tract, and immunohistochemical staining was taken to investigate the influence of Mg-6Zn alloy on tight junction of peri-implant tissues.
2 Experimental
2.1 Materials and extract preparation
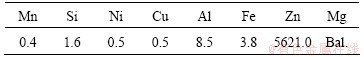
The extruded Mg–6Zn alloy, prepared with high purity Mg (≥99.99%) (Xinxiang Jiuli Magnesium Co., Ltd, Henan Province, China.) and high purity Zn (≥99.999%) (MCC Huludao Nonferrous Metals Group Co., Ltd, Liaoning Province, China), was generously donated by the school of Material Science and Engineering in Shanghai Jiao Tong University. The materials were prepared as previously described [13], and the composition of this alloy is presented in Table 1. The as-extruded Mg-6Zn rods were processed into disc samples and pin-shaped samples which were tested in the experiment. The disc samples with a diameter of 11.3 mm and a height of 2.0 mm were used for the in vitro cell experiments. The pin-shaped samples with dimensions of 3.8 mm × 3 mm × 0.26 mm were designed to be implanted into the intestinal tract of rabbits. Prior to testing, all the samples were cleaned ultrasonically by acetone and ethanol and finally sterilized with 29 kGy of cobalt-60 radiation.
Table 1 Chemical composition of Mg-6Zn alloy (mass fraction, 10-5)
Medical Ti was used. The Ti pins were cut from Ti stapling pins (TLC series) (Ti-3Al-2.5V Alloy, Ethicon Endo-Surgery, Inc.), and cleaned ultrasonically in acetone, ethanol and distilled water before the experiment.
Extracts were prepared according to ISO 10993-5. The cell culture medium, Dulbecco’s modified Eagle’s medium high glucose (DMEM, Gibco; code 11965-092), supplemented with 10% fetal bovine serum (FBS, Gibco; code 16000044) was placed over the disc samples at room temperature for the surface area to extraction medium ratio of 1.25 cm2 /mL, and they were extracted in a humidified atmosphere with 5% CO2 at 37 °C for 24 h. Then, the extracts were pooled and refrigerated at 4 °C. To observe a dose-response relationship, the extracts were serially diluted with DMEM to different concentrations (40% and 20%). The concentrations of released Mg and Zn ions of 40% Mg-6Zn alloy extracts were 6.34±1.75 and 0.0052±0.0009 mmol/L, respectively, detected by the ICP-AES (inductively coupled plasma- atomic emission spectroscopy).
2.2 Animal model and study design
The animal experiment was authorized according to the Guidance Suggestions for the Care and Use of Laboratory Animals (issued by the Ministry of Science and Technology of the People’s Republic of China), and were approved by the Ethnics Committee of the Affiliated 6th Hospital of Shanghai Jiao Tong University. The animals were supplied by the Sino-British Sippr/BK Lab Animal Ltd, Co, China (License No: SCXK (hu) 2008-0016). In the in vivo experiment, 63 adult New Zealand rabbits with a mean body mass of (2.35±0.5) kg were used in the experiments, randomly and equally assigned to three groups. The rabbits were placed under general anesthesia by the intravenous administration of sodium pentobarbital at a dose of 30 mg per kilogram of body mass. All surgical procedures were carried out under sterile conditions. Through a midline laparotomy, the small intestine was exposed and intestinal anastomosis was implemented using the side-side stapler (with Mg-6Zn alloy nails and titanium nails) at the site of 40 cm from the ligament of Traitz. A size of the anastomotic stoma was ensured 5 cm. Then, the abdomen was closed layer by layer. In the control group, nothing was implanted into the intestinal tract of 21 rabbits, and the incision was sutured using the 4-0 absorbable suture. In the second group of 21 rabbits, the nails of Mg-6Zn alloy were implanted into the intestinal tract, which was called the Mg-6Zn alloy group. In another group with 21 rabbits, titanium nails were implanted, which was called the titanium group. In all the three groups, seven rabbits were sacrificed postoperatively at 1, 2 and 3 weeks, respectively.
2.3 mRNA isolation and real-time polymerase chain reaction (PCR)
IEC-6 cells were purchased (Institute of Zoology, CAS, Kunming, China) and cultured in Dulbecco’s modified Eagle medium high glucose (DMEM, Gibco; code 11965-092), supplemented with 10% fetal bovine serum (FBS, Gibco; code 16000044), 100 IU/mL penicillin and 100 IU/mL streptomycin in a humidified incubator at 95% relative humidity and 5% CO2 at 37 °C. The cells were cultured as described by the previous studies [8]. Briefly, IEC-6 cells were cultured on different concentrations (0, 20% and 40%) of Mg-6Zn alloy extracts for different time periods (1, 3 and 5 d), respectively. Total RNA was extracted from cell layers using a Trizol reagent (Invitrogen, Carlsbad, CA) in accordance with the manufacturer’s instructions. The concentration of RNA was measured using a Nano Drop ND-1000 Spectrophotometer (Nano Drop Technologies, Wilmington, USA) and the purity (A260/A280) greater than 1.8 was used. Complementary DNA (cDNA) was synthesized using the PrimeScriptTM RT reagent Kit (TaKaRa Biotechnology, Dalian, China), according to the manufacturer’s instructions. In brief, RNA (400 ng) was transcripted in a volume containing 5 × PrimeScriptTM buffer (2 μL), PrimeScriptTM RT Enzyme Mix I (0.5 μL), 50 μmol/L Oligo dT Primer (0.5 μL) and 100 μmol/L Random 6 mers (0.5 μL) in a PTC-200 Peltier Thermal Cycler (MJ Research, Watertown, MA), and RNase-free water was added to get total volume of 10 μL. The reaction mixture was incubated at 37 °C for 15 min for reverse transcription, then, the reverse transcriptase was inactivated at 85 °C for 5 s. Primers were designed (Table 2) and synthesized (Takara Biotechnology Co., Ltd., Dalian, China). Real-time PCR was performed using a quantitative real-time amplification system (Light Cycler instrument, Roche Diagnostic, Germany). The SYBR Premix Ex Taq TM kit (TaKaRa Biotechnology, Dalian, China) was used in each reaction. Briefly, cDNA (2 μL) reacted with 10 μL 2× SYBR
Premix Ex Taq TM kit (TaKaRa Biotechnology, Dalian, China) was used in each reaction. Briefly, cDNA (2 μL) reacted with 10 μL 2× SYBR Premix Ex Taq TM buffer, 0.5 μL of 10 μmol/L each primer and 7.2 μL dH2O in a 20 μL final reaction volume. PCR conditions were as follows. The initial denaturation was handled at 95 °C for 30 s, followed by 40 PCR cycles (95 °C for 30 s, 62 °C for 20 s).
Premix Ex Taq TM buffer, 0.5 μL of 10 μmol/L each primer and 7.2 μL dH2O in a 20 μL final reaction volume. PCR conditions were as follows. The initial denaturation was handled at 95 °C for 30 s, followed by 40 PCR cycles (95 °C for 30 s, 62 °C for 20 s).
2.4 Immunohistochemical analysis
At predetermined time, the rabbits were sacrificed, and the tissue samples of 5 cm2 surrounding the implants were fixed in 10% buffered formaldehyde. Immunohisto- chemical analysis was conducted to investigate the expression of tight junction related genes. Tissue sections were deparaffinized in xylene, and then rehydrated in graded concentrations of ethyl alcohol (100%, 95% and 75%, then water). The sections were then microwave treated twice in citrate buffer (pH value of 6.0) at 99 °C for 6 min. After the sections were placed in 3% H2O2 for 10 min to inhibit the endogenous peroxide activity, they were washed three times with phosphate-buffered saline (PBS) buffer for 5 min and placed in normal mouse serum as blocking antibody at room temperature for 10 min. The sections were evaluated by antibody for Occludin (SC-8144, Santa) and ZO-1(BA-2825, boster). After incubation at 4 °C for 24 h, sections were washed three times with PBS buffer for 10 min. Biotinylated anti-mouse/rabbit immunoglobulin was used as the second antibody. 3,3-Diaminobenzidine tetrahydro- chloride (DAB) was used as a chromogen. The sections were evaluated in the light microscope using the MICRO IMAGETM Software (Olympus Optical Corp. Ltd., Tokyo, Japan). The expression of all tight junction relative indicators was examined by the number of positive cells using the image analysis software (Image-Pro Plus, Media Cybernetics, USA).
2.5 Statistical analysis
Statistical analysis was performed with the SPSS 18.0 Software Package (SPSS Inc., Chicago, USA). The experimental values were analyzed using the paired- samples t test and were expressed by the mean values ± standard deviation (SD). Then, one-way ANOVA analysis was performed to determine differences between groups for each evaluated parameter that was evaluated at each time point. Non parametrical tests [κ independent samples tests (Kruskal–Wallis test)] were calculated when equal variances were not assumed in one-way ANOVA. The level of significance was defined as P<0.05.
Table 2 Real-time PCR primer sequence of target genes

3 Results
3.1 General condition of experimental animals
A total of 63 rabbits were included in the final analysis without dropping out. All animals survived until the completion of the study and had stable body mass. The rabbits in each group grew well and no postoperative adverse effects such as infection, pyogenesis and body fluid effusion occurred throughout the experimental periods.
3.2 In vitro real-time PCR quantification of Occludin and ZO-1 gene expression
The mRNA expression levels of Occludin and ZO-1 were detected by real-time PCR. Figure 1 shows the mRNA expression levels of Occludin and ZO-1 in IEC-6 cells cultured in the extraction media for periods of 1, 3 and 5 d. There is no statistic difference between the extract groups and the control group at 1 d in the expression of ZO-1. After 3 d, the expression of ZO-1 in the 40% extract group is significantly higher than that in the control group and in the 20% extract group (P<0.05), while there is no statistic difference observed between the 20% extract group and the control group. After 5 d, the expression of ZO-1 in both extract groups is significantly higher than that in the control group (P<0.05), while there is no statistic difference observed between the two extract groups. For Occludin mRNA expression, no significant difference is observed between the 20% extract group and the control group at 1 d. While after cultured for 3 and 5 d, the expression levels in both extract groups significantly increase as compared with that in the control group (P<0.05). Though the expression of Occludin in 40% extract group is significant higher than that in the 20% extract group after 3 d (P<0.05), there is no significant difference observed between the two extract groups after 5 d.
3.3 In vivo immunohistochemical evaluation
Figure 2 shows representative immunohistochemical pictures depicting the experiment of Occludin and ZO-1 genes in the peri-implant’s intestine tissues. Figures 3(a) and (b) show the statistical results of the immunohisto- chemical analysis. The results show that the number of Occludin and ZO-1 positive cells in intestine in the Mg-6Zn alloy groups is significant higher than that in the control groups and in the titanium groups at the three points of 1, 2 and 3 weeks post-operation (P<0.05).
4 Discussion
Epithelia not only form barriers against unlimited passage of solutes and water, but also regulate and allow distinct permeation across that barrier. The pathways are located in different sites. Within the cell membranes, the pathways are formed via channels, carriers and transporting ATPases, while the paracellular pathways between the cells are sealed against uncontrolled passage by the tight junction (TJ). TJs are cell–cell adhesion structures located at the uppermost portion of lateral membrane at the limit with the apical surface [14]. They are required for cell adhesion and paracellular barrier functions, and are now thought to be partly involved in fence functions and in cell polarization. The TJ dysfunction would cause the dysequilibrium of the paracellular transport of solutes and water, increase the permeability to large molecules, and represent both the reason and consequence of the disease [15-17]. TJs are integrated by a complex group of molecules that include integral and adaptor proteins [18]. Integral proteins such as Occludin establish cell to cell interactions that seal the intercellular space and are responsible for the paracellular ionic selectivity. Adaptor proteins such as ZOs could act as a cytoplasmic platform that concentrates a variety of cell signaling proteins at the TJ, including kinases, G proteins and transcription factors.
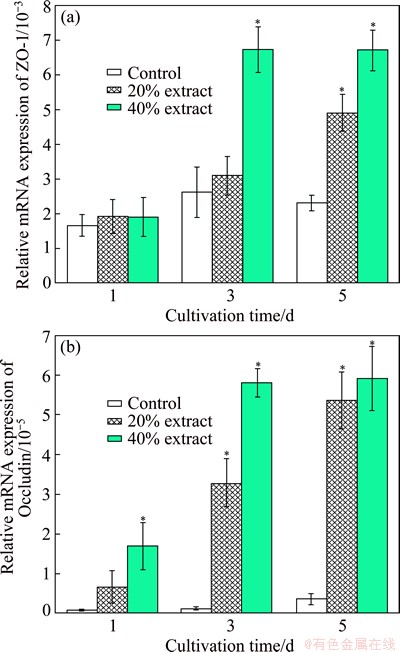
Fig. 1 Relative mRNA expression levels of ZO-1 (a) and Occludin (b) in IEC-6 cells cultured in extraction media for periods of 1, 3 and 5 d
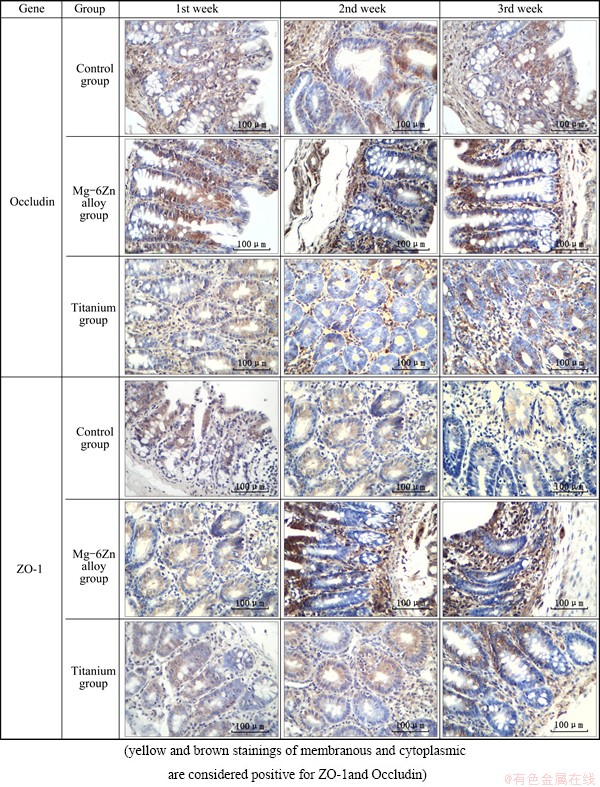
Fig. 2 Results of immunohistochemical staining of ZO-1 and Occludin
The in vitro study shows that Mg-6Zn alloy extracts with a low concentration (20%) can promote the expression of TJ relative genes, while along with the increase of Mg-6Zn concentration, the effect is much more obvious. The expression levels of Occludin and ZO-1 cultured in Mg-6Zn alloy extracts with a 20% concentration are higher compared with those in the control group after 5 d. When the concentration reaches 40%, the expression of both TJ relative genes increases, as compared with the control group. These results may suggest that 20% and 40 % extracts of Mg-6Zn alloy can promote the regeneration of tight junction between cells and the recovery of the barrier function. The in vivo experiment results show that when Mg-6Zn alloy is implanted in intestine, the expression levels of TJ relative genes in peri-implant’s tissues are significantly higher than those in the titanium groups and the control groups. The outcomes are consistent with the in vitro results.
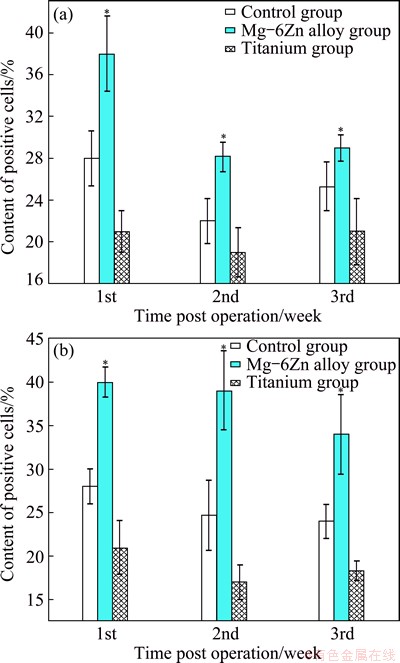
Fig. 3 Statistical results of immunohistochemical analysis of expression of Occludin (a) and ZO-1 (b)
The mechanism of the increasing expression of TJ relative genes is complex. There are some possible explanations. First, Mg is essential microelement in human body and critical for many cellular functions [19]. As displayed above, the concentration of Mg2+ in the extracts is much higher than that in the normal culture medium. Though, the corrosion rate of Mg-6Zn alloy in vivo is different from that in vitro, the local concentration of Mg2+ is speculated to be also higher than that in the normal tissue. In Ref. [20], the results showed that the extracellular Mg2+ could regulate the expression and localization of tight junction related claudin-16. The results of another experiment showed that the Mg deficiency induced lower mRNA content of factors (ZO-1, Occludin) controlling gut barrier function in the ileum [21]. According to the studies, the increased expression levels of tight junction relative genes in this research are speculated to be associated with the high concentration of extracellular Mg2+. Second, another main influence factor is the variation of Zn2+ concentration. Zn is one of the trace elements with a critical biochemical role in the human body. It is also essential to over 300 DNA-binding proteins and over 2000 Zn-related transcription factors [22]. To evaculate the effect of high level of zinc oxide on intestinal mucosal barrier in piglet, increased expression levels of Occludin and ZO-1 were detected [23]. However, in our research, the concentration of released Zn ions of 40% Mg-6Zn alloy extracts is only (0.0052±0.0009) mmol/L. The effect of Zn ions on tight junction is speculated to be very weak at such a low concentration. HU et al [24] found that the supplementation with ZnO at 500 mg Zn per kilogram of body mass had no influence on the function of the intestinal barrier. However, the role and mechanism of Mg and Zn ion alone on tight junction still need further studies.
5 Conclusions
1) The in vitro experiments were performed to investigate the effect of Mg-6Zn alloy on tight junction of IEC-6 cells. Mg-6Zn extract with a low concentration can induce the expression of tight junction relative genes (Occludin, ZO-1) in vitro.
2) The in vivo experiments were performed when Mg-6Zn alloy staples were implanted into intestine, and increased expression of related tight junction genes (Occludin, ZO-1) were observed. The results show that the Mg-6Zn alloy may promote the regeneration of tight junction and the development of barrier function.
References
[1] DONATO T A G, de ALMEIDA L H, NOGUEIRA R A, NIEMEYER T C, GRANDINI C R, CARAM R, SCHNEIDER S G, SANTOS Jr A R. Cytotoxicity study of some Ti alloys used as biomaterial [J]. Materials Science and Engineering C, 2009, 29(4): 1365-1369.
[2] SHAHRI S, IDRIS M H, JAFARI H. Effect of solution treatment on corrosion characteristics of biodegradable Mg-6Zn alloy [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(5): 1490-1499.
[3] GINSBERG G, COPE C, SHAH J, MARTIN T, CARTY A, HABECKER P, KAUFMANN C, CLERC C, NUUTINEN J, TORMALA P. In vivo evaluation of a new bioabsorbable self-expanding biliary stent [J]. Gastrointestinal Endoscopy, 2003, 58(5): 777-784.
[4] ZHANG Shao-xiang, ZHANG Xiao-nong, ZHAO Chang-li. Research on an Mg-Zn alloy as a degradable biomaterial [J]. Acta Biomaterialia, 2010, 6(2): 626-640.
[5] ZHANG Shao-xiang, LI Jia-nan, SONG Yang. In vitro degradation, hemolysis and MC3T3-E1 cell adhesion of biodegradable Mg-Zn alloy [J]. Materials Science and Engineering C, 2009, 29(6): 1907-1912.
[6] CHEN Dao-yun, HE Yao-hua, TAO Hai-rong, ZHANG Yan. Biocompatibility of magnesium-zinc alloy in biodegradable orthopedic implants [J]. International Journal of Molecular Medicine, 2011, 28(3): 343-348.
[7] WANG Zhan-hui, YAN Jun, ZHENG Qi. Effects of biodegradable Mg-6Zn alloy extracts on cell cycle of intestinal epithelial cells [J]. Journal of Biomaterials Applications, 2013, 27(6): 739-747.
[8] WANG Zhan-hui, YAN Jun, ZHENG Qi. Effects of biodegradable Mg-6Zn alloy extracts on apoptosis of intestinal epithelial cells [J]. Materials Science and Engineering B, 2012, 177(4): 388-393.
[9] POWELL D W. Barrier function of epithelia [J]. American Journal of Physiology, 1981, 241: 275-288.
[10] ANDERSON J M, VANITALLIE C M, FANNING A S. Setting up a selective barrier at the apical junction complex [J]. Current Opinion in Cell Biology, 2004, 16(2): 140-145.
[11] ZEISSIG S, BURGEL N, GUNZEL D, RICHTER J, MANKERTZ J. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease [J]. Gut, 2007, 56(1): 61-72.
[12] SCHUMANN M, KAMEL S, PAHLITZSCH M L, LEBENHEIM L. Defective tight junctions in refractory celiac disease [J]. Annals of the New York Academy of Sciences, 2012, 1258: 43-51.
[13] ZHANG Shao-xiang. Research on an Mg-Zn alloy as a degradable biomaterial [J]. Acta Biomaterialia, 2010, 6(2): 626-640.
[14] FARQUHAR M G, PALADE G E. Junctional complexes in various epithelia [J]. Journal of Cell Biology, 1963, 17: 375-412.
[15] AL-SADI R, KHATIB K, GUO S, YE D, YOUSSEF M, MA T. Occludin regulates macro-molecule flux across the intestinal epithelial tight junction barrier [J]. American Journal of Physiology- Gastrointestinal and Liver Physiology, 2011, 300(6): 1054-1064.
[16] SANDLE G I. Pathogenesis of diarrhea in ulcerative colitis: New views on an old problem [J]. Journal of Clinical Gastroenterology, 2005, 39(4,Suppl 2): s49-s52.
[17] SIMON D B, LU Y, CHOATE K A, VELAZQUEZ H. Paracellin-1, a renal tight junction protein required for paracellular Mg2+-resorption [J]. Science, 1999, 285(5424): 103-106.
[18] GONZALEZ-MARISCAL L, BETANZOS A, NAVA P, JARAMILLO B E. Tight junction proteins [J]. Progress in Biophysics & Molecular Biology, 2003, 81(1): 1-44.
[19] WU N, VEILLETTE A. Immunology: Magnesium in a signaling role [J]. Nature, 2011, 475(7357): 462-463.
[20] AKIRA I, KEISHI K, KOSUKE A. Extracellular Mg2+ regulates the tight junctional localization of claudin-16 mediated by ERK- dependent phosphorylation [J]. Biochimica et Biophysica Acta, 2010, 1798(3): 415-421.
[21] BARBARA D P, AUDREY M N, LOUISE D. Changes in intestinal bifidobacteria levels are associated with the inflammatory response in magnesium-deficient mice [J]. Journal of Nutrition, 2010, 140(3): 509-514.
[22] HO E. Zinc deficiency, DNA damage and cancer risk [J]. Journal of Nutritional Biochemistry, 2004, 15(10): 572-578.
[23] HU Cai, QIAN Zhong-cang, LIU Hai, XU Yong. Effect of high level of zinc oxide on tight junction protein expression in intestinal epithelial cells and intestinal mucosal barrier in early weaning piglets [J]. Acta Veterinaria et Zootechnica Sinica, 2009, 40 (11): 1638-1644.
[24] HU Cai-hong, SONG Juan, LI Ya-li, LUAN Zhao-shuang, ZHU Kang. Diosmectite–zinc oxide composite improves intestinal barrier function, modulates expression of pro-inflammatory cytokines and tight junction protein in early weaned pigs [J]. British Journal of Nutrition, 2013, 110: 681-688.
于 嵩1,王啸虎1,2,陈义刚3,郑 起1,2,张小农4,赵常利4,张绍翔5,阎 钧1
1. 上海交通大学 附属第六人民医院 普通外科,上海 200233;
2. 上海交通大学 医学院,上海 200025;
3. 南京医科大学 附属无锡市第二人民医院 普通外科,南京 214002;
4. 上海交通大学 材料学院 金属基复合材料国家重点实验室,上海 200240;
5. 苏州奥芮济医疗科技有限公司,苏州 215513
摘 要:研究Mg-6Zn合金对肠上皮紧密连接的影响。体外实验中,肠上皮细胞(IEC-6)分别在不同浓度(0,20%和40%)合金浸提液中培养1、3和5天。逆转录聚合酶链反应(RT-PCR)法检测结果显示,当IEC-6细胞在40%和20%浓度合金浸提液中培养时,Occludin和ZO-1 mRNA表达水平在各不同的时间点相对于对照组升高。体内实验中,镁合金吻合钉和医用钛吻合钉分别植入兔小肠中1、2和3个星期,采用免疫组织化学染色法检测植入部位周围组织紧密连接相关蛋白的表达。结果显示,植入后第1、2和3星期三个时间点,Mg-6Zn合金组植入部位周围组织中ZO-1、Occludin表达水平明显高于钛钉组和对照组。综上所述,Mg-6Zn合金植入肠道后可促进肠道周围组织紧密连接的再生,其浸提液在一定浓度下可诱导细胞紧密连接相关基因的表达。
关键词:Mg-6Zn合金;肠上皮细胞; 紧密连接
(Edited by Mu-lan QIN)
Foundation item: Project (30901422) supported by the National Natural Science Foundation of China; Project (YG2010MS45) supported by Shanghai Jiao Tong University Interdisciplinary (Biomedical Engineering) Research Fund, China; Project (09XJ21005) supported by School of Medicine Science and Technology Fund, Shanghai Jiao Tong University, China
Corresponding author: Jun YAN; Tel: +86-21-64269181; E-mail: shanghaijunyan007@163.com
DOI: 10.1016/S1003-6326(15)64014-6

