
Thermal stability and mechanical properties of Gd-Co-Al bulk glass alloys
CHEN Ding(陈 鼎)1, 2, A. Takeuchi2, A. Inoue2
1. Institute for Metal Research, Hunan University, Changsha 410082, China;
2. Institute for Materials Research, Tohoku University, Sendai 980-8577, Japan
Received 15 July 2007; accepted 10 September 2007
Abstract: The glass forming ability of Gd-Co-Al ternary alloy systems with a composition ranging from 50% to 70% (molar fraction) for Gd and from 5% to 40% (molar fraction) for Al were investigated by copper mold casting and Gd60Co25Al15 bulk glass alloy cylinders with the maximum diameter of 5 mm were obtained. The reduced glass transformation temperature (Tg/Tm) and the distance of supercooling region ?Tx are 0.616 and 45 K, respectively for this Gd-Co-Al alloy. The compressive fracture strength (σf) and elastic modulus (E) of Gd-Co-Al glassy alloys are 1 170-1 380 MPa and 59-70 GPa, respectively. The Gd-Al-Co bulk glassy alloys with high glass forming ability and good mechanical properties are promising for the future development as a new type function materials.
Key words: bulk metallic glass; Gadolinium-cobalt-aluminum alloy; glass-forming ability; mechanical property
1 Introduction
Since the first RE-based bulk metallic glass system was discovered in Ln-TM-Al (Ln=lanthanide metals, TM=Ⅵ-Ⅷ group transition metal) alloy system by INOUE et al[1-2], a number of investigations on the glass-forming ability(GFA), structure and properties were made for various light rare earth(RE)-based alloy systems and some kinds of light RE-based bulk amorphous alloys/bulk metallic glasses(BMG) were obtained[3-10]. For instance, Nd/Pr-based bulk amorphous alloys with hard magnetic properties at room temperature were obtained by INOUE et al[3-5], Sm- and Y-based bulk metallic glasses were developed by FAN et al[6] and GOU et al[7]. ZHANG et al[8] prepared the Ce-based bulk metallic glasses with polymerlike thermoplastic behavior caused by their extraordinary low glass transition temperature Tg, which is similar to or lower than that of many polymers. Furthermore, other kinds of RE-based BMGs, i.e., the heavy RE-based alloy systems, were obtained mainly by WANG et al[9]. Among these new heavy RE-based BMGs, the Gd-based BMG is worthy to note for its high thermal stability and unique properties (e.g. magnetic and magneto-optical properties), which are attractive for application as functional materials and some Gd-based BMGs were obtained by LI et al[10-11]. Just almost at the same time, we have successfully synthesized a series of Gd-TM-Al (TM=Fe, Co and Ni) ternary bulk amorphous and bulk metallic glasses[12-14]. In this work, some Gd-Co-Al bulk metallic glasses with great glass forming ability properties were obtained by copper mold casting and their mechanical properties were investigated.
2 Experimental
The master alloys of Gd-Co-Al alloy systems with composition range of 50%-70%Gd (molar fraction, the same below) and 5%-40%Al were prepared by induction-melting a mixture of pure Gd, Co and Al metals in an argon atmosphere. The amorphous ribbons with a thickness of about 20-30 μm and a width of 1mm were produced by a single-roller melt spinning method in an argon atmosphere. Based on fundamental data of the Gd-Co-Al amorphous alloy ribbons, the bulk metallic glasses of Gd50-70Co5-40Al5-35 (molar fraction, %) were prepared as cylindrical samples with a length of about 50 mm and diameters ranging from 1 to 6 mm by injection casting of the molten alloy into copper molds with cylindrical cavities. The structure of the as-cast cylindrical samples was examined by X-ray diffractometry. The thermal stability associated with crystallization and melting was measured by differential scanning calorimetry(DSC) at a heating rate of 0.67 K/s. Magnetic properties were measured with a vibrating sample magnetometer(VSM) under an applied field of 1 432 kA/m at room temperature. Compressive testing was performed with an Instron testing machine at a strain rate of 5×10-4 s-1. The gauge dimensions of the specimen were 2 mm in diameter and 4 mm in height. The fracture face of the compression samples were observed by scanning electron microscopy(SEM).
3 Results and discussion
Fig.1 shows the composition ranges in which an amorphous phase can form in Gd-Co-Al ternary alloy systems by copper mold casting. From this figure, Gd-Co-Al BMGs with the maximum diameter of 5 mm are obtained in Gd-rich composition regions.
Fig.2 shows the XRD patterns of the as-cast

Fig.1 Composition ranges of Gd-Co-Al BMGs with different diameters

Fig.2 XRD patterns of as-cast Gd60Co25Al15 cylinders with different diameters together with data of amorphous ribbons shown for comparison
Gd60Co25Al15 cylinders up to 6 mm in diameter, which shows the highest glass-forming ability in Gd-Co-Al system. As seen in Fig.2, the broad diffraction peaks confirm for Gd60Co25Al15 glassy alloys with the diameter from 1 to 5 mm. These broad diffraction peaks indicate the formation of an amorphous phase in these as-cast rods. Therefore, the maximum diameter of the BMGs is up to 5 mm for Gd60Co25Al15 glassy alloy.
Fig.3 shows the DSC curves of the as-cast Gd-Co-Al bulk amorphous alloy bulk metallic glass cylinders with a diameter of 1mm. The contents of Al and Gd are fixed at 15% (Fig.3(a)) and 60% (Fig.3(b)), respectively. The thermal stability of these Gd-Co-Al bulk amorphous alloys bulk metallic glasses is listed in Table 1. As shown in Fig.3 and Table 1, most of the Gd-Co-Al bulk amorphous samples exhibit the glass transition temperature(Tg), therefore, it can be said that these amorphous alloys can mostly form as bulk metallic glasses. And the glass transition temperature (Tg), the crystallization temperature(Tx) and melting temperature (Tm) are 572, 617 and 928 K for the Gd60Co25Al15 glassy alloy. So the supercooled liquid region (?Tx) and the reduce glass transition temperature Trg, which is defined

Fig.3 DSC curves of as-cast Gd-Co-Al bulk amorphous alloys/ bulk metallic glasses cylinders with diameter of 1 mm: (a) Gd85-xCoxAl15 (x=35, 30, 25, 20, 15); (b) Gd60Co40-xAlx (x=5, 10, 15, 20, 25)
Table 1 Thermal stability of as-cast Gd-Co-Al cylinders with diameter of 1 mm
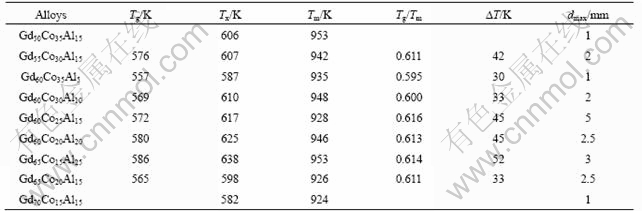
Tx is cystallization temperature; Tm is melting temperature; Tg is glass transition temperature; Tg/Tm is reduced galss transition temperature; ?T=Tx-Tg; dmax is maximum diamater from the ratio of Tg/Tm are 45 K and 0.611 for Gd60Co25Al15 glassy alloy. Obviously, compared with the values of ?Tx and Trg of the typical BMG alloy systems, in which the former is normally higher than 50 K and the latter is near 2/3[15-17], and the Gd-based BMGs of this study is smaller. The reason for the high glass-forming ability of the Gd-based alloys that have lower ?Tx and Trg is not clear yet. It is possible to that the alloy composition is near the eutectic point and the Gd element has an excellent ability to purify the melt and further to improve the glass-forming ability of the present alloys.
The data of the mechanical properties of these Gd-based BMGS are listed in Table 2. As shown in Table 2, the compressive fracture strength of the Gd-Co-Al bulk metallic glasses decreases from 1 300 to 1 170 MPa with decreasing Co content from 30% to 20% while it increases from 1 180 to 1 380 MPa with increasing Al content from 10% to 25%. It should be noted that the increase of the compressive fracture strength with Al content in the Gd-Co-Al bulk metallic glassy systems is consistent with that in Ln-Ni-Al and Ln-Cu-Al bulk metallic glasses systems[1-2]. In addition, it is noted that the Gd-based BMGs exhibit the highest fracture strength among the RE (rare-earth)- based bulk metallic glasses reported to date: the previous data show lower fracture strength below 1 000 MPa [1-2, 18]. Furthermore, the mechanical properties of Gd-Based BMG would be improved further by adding the other elements. The high glass-forming and good mechanical properties of Gd-based BMG will offer the potential application as new kind of function material and device.
Fig.4 shows the SEM images of the fracture surface of the as-cast Gd60Co25Al15 bulk metallic glass rod. It can be seen from Fig.4(a) that the fracture surface shows a number of small fracture zones with their zone planes appearing with angles of 60?-90? to the direction of applied load. The fracture behavior is different from the previous results for the Zr-, Cu- and Ni-based bulk metallic glasses, in which the fracture occurs only along the maximum shear plane with an angle of 45? to the direction of applied load[19-21]. On the contrary, the fracture behavior is similar to that of the Fe-based bulk metallic glass[22-23]. The simultaneous generation of a number of small fracture zones is presumably due to the easy initiation of fracture at many sites. The easy initiation results from the high stress level and the shock wave caused by its initiation, which can induce to crack at different sites because of high fracture stress level due to strong bonding forces among the constituent elements. The fracture mechanism is also supported from the micrographs of the fracture surface with characteristic of considerably fine shell pattern and the generation of the propagation of the crack, both of which are shown in Fig.4(b). On the other hand, it is confirmed that the fracture specimen shows river pattern on the surface (Fig.4(c)) and vein pattern (Fig.4(d)), the latter of which is an enlarged SEM micrograph taken from a part of the specimen marked with circle combined with symbol “c” in Fig.4(b). However, no shear band can be observed in the Gd-Co-Al bulk metallic glasses before and after fracture.
Table 2 Mechanical properties of Gd-Co-Al bulk metallic glasses with diameter of 2 mm


Fig.4 SEM images of fracture surface of cast Gd60Co25Al15 bulk metallic glass rod with diameter of 2 mm
4 Conclusions
1) The Gd-Co-Al bulk metallic glasses were obtained by the copper mold casting method. The maximum diameter for Gd60Co25Al15 glass alloy is 5 mm.
2) The Gd-Co-Al bulk metallic glasses have high glass-forming ability and thermal stability. The reduced glass transformation temperature (Tg/Tm) and the distance of supercooling region ?Tx are 0.616 and 45 K respectively for the Gd60Co25Al15 glassy alloys.
3) The Gd-Co-Al bulk metallic glasses exhibit good mechanical properties. The compressive fracture strength and elastic modulus of the Gd-Co-Al bulk metallic glasses are 1 170-1 380 MPa and 59-70 GPa, respec- tively. The Gd-Co-Al bulk metallic glasses exhibit the highest compressive fracture strength among the other available RE-based bulk metallic glasses found up to date with keeping the trends of the conventional bulk metallic glasses
References
[1] INOUE A, KITA K, ZHANG T, MASUMOTO T. An amorphous La55Al25Ni20 alloy prepared by water quenching [J]. Mater Trans, JIM, 1989, 30(9): 722-725.
[2] INOUE A, ZHANG T, MASUMOTO T. Production of amorphous cylinder and sheet of La55Al25Ni20 alloy by a metallic mold casting method. [J]. Mater Trans, JIM, 1990, 31(5): 425-428.
[3] INOUE A, ZHANG T, ZHANG W, TAKEUCHI A. Bulk Nd-Fe-Al amorphous alloys with hard magnetics properties [J]. Mater Trans, JIM, 1996, 37(2): 99-108.
[4] INOUE A, ZHANG T, TAKEUCHI A, ZHANG W. Hard magnetic bulk amorphous Nd-Fe-Al alloys of 12 mm in diameter made by suction casting [J]. Mater Trans, JIM, 1996, 37(4): 636-640.
[5] INOUE A, ZHANG W, TAKEACHI A. Preparation of bulk Pr-Fe-Al amorphous alloys and characterization of their hard magnetic properties [J]. Mater Trans JIM, 1996, 37(12): 1731-1740.
[6] FAN G J, LOSER W, ROTH S, ECKERT J.  Glass-forming ability of RE-Al-TM alloys (RE=Sm, Y TM=Fe, Co, Cu) [J]. Acta Mater, 2000, 48(15): 3823-3831.
Glass-forming ability of RE-Al-TM alloys (RE=Sm, Y TM=Fe, Co, Cu) [J]. Acta Mater, 2000, 48(15): 3823-3831.
[7] GUO F Q, POON S J, SHIFLET G J. Metallic glass ingots based on yttrium [J]. Appl Phys Lett, 2003, 83(13): 2575-2577.
[8] ZHANG B, ZHAO D Q. PAN M X, WANG W H, GREER A L. Amorphous metallic plastic [J]. Phys Rev Lett, 2005, 94, 205-502.
[9] LI S, XI X K, WEI Y X, LUO Q, WANG Y T, TANG M B, ZHANG B, ZHAO Z F, WANG R J, PAN M X, ZHAO D Q, WANG W H. Formation and properties of new heavy rare-earth-based bulk metallic glasses [J]. Sci and Tech Adv Mater, 2005, 6: 823-827.
[10] LI S, ZHAO D Q, PAN M X, WANG W H. A bulk metallic glass based on heavy rare earth gadolinium [J]. J Non-Cryst Solids, 2005, 351(30/32): 2568-2571.
[11] LI S, WANG R J, PAN M X, ZHAO D Q, WANG W H. Heavy rare earth based bulk metallic glasses with high thermal stability [J]. Intermetallics, 2006, 14(6): 592-595.
[12] CHEN D, TAKEUCHI A, INOUE A. Thermal stability and magnetic properties of Gd-Fe-Al bulk amorphous alloys [J]. J Alloy Com, 2007, 440: 199-203.
[13] CHEN D, TAKEUCHI A, INOUE A. Gd-Co-Al and Gd-Ni-Al bulk metallic glasses with high glass forming ability and good mechanical properties [J]. Mater Sci Eng A, 2007, 457: 226-230.
[14] CHEN D, TAKEUCHI A, INOUE A. Gd-Ni-Al bulk glass alloys with great glass forming ability and good mechanical properties [J]. Journal of Materials Science, 2007, 42(10): 8662-8666.
[15] INOUE A. Stabilization of metallic supercooled liquid and bulk amorphous alloys [J]. Acta Mater, 2000, 48(1): 279-306.
[16] JOHNSON W L. Bulk amorphous metal-an emerging engineering material [J], JOM, 2002, 54(3): 40-43.
[17] WANG W H, DONG C, SHEK C H. Bulk metallic glasses [J]. Mater Sci Eng R, 2004, 44: 45-89.
[18] BIAN Z, INOUE A. Ce-Cu-Fe-Al-Si bulk metallic glass alloys with high glass forming ability [J]. Mater Trans, 2005, 46(11): 2541-2544.
[19] INOUE A, ZHANG T. Fabrication of bulky zr-based glassy alloys by suction casting into copper mold [J] Mater Trans JIM, 1995, 36(9): 1184-1187.
[20] INOUE A, ZHANG W, ZHANG T, KUROSAKA K. High-strength Cu-based bulk glassy alloys in Cu-Zr-Ti and Cu-Hf-Ti ternary systems [J]. Acta Mater, 2001, 49(14): 2645-2652.
[21] ZHANG T, INOUE A. New bulk glassy Ni-based alloys with high strength of 3 000 MPa [J]. Mater Trans, 2002, 43(4): 708-711.
[22] INOUE A, SHEN B L, YAVARI A R, GREER A L.  Mechanical properties of Fe-based bulk glassy alloys in Fe-B-Si-Nb and Fe-Ga-P-C-B-Si systems [J]. J Mater Res, 2003, 18(6): 1487-1492.
Mechanical properties of Fe-based bulk glassy alloys in Fe-B-Si-Nb and Fe-Ga-P-C-B-Si systems [J]. J Mater Res, 2003, 18(6): 1487-1492.
[23] INOUE A, SHEN B L, CHANG C T. Super-high strength of over 4000 MPa for Fe-based bulk glassy alloys in [(Fe1-xCox)0.75B0.2- Si0.05]96Nb4 system [J]. Acta Mater, 2004, 52(14): 4093-4040.
(Edited by LI Xiang-qun)
Corresponding author: CHEN Ding; Tel: +86-731-8821648; E-mail:ma97chen@hotmail.com