Al-Si-Mg-Er合金的热稳定性和析出相的显微组织
来源期刊:中国有色金属学报(英文版)2021年第1期
论文作者:何忆 席海辉 明文全 邵秦秦 沈若涵 赖玉香 伍翠兰 陈江华
文章页码:1 - 10
关键词:Al-Si-Mg合金;Er;自然时效;析出相;热稳定性
Key words:Al-Si-Mg alloy; Er; natural aging; precipitate; thermal stability
摘 要:采用显微硬度计和透射电镜研究Er元素对热处理Al-Si-Mg合金显微硬度和析出行为的影响。作为对照,同时研究人工时效前引入自然时效对合金硬度和析出行为的影响。研究结果表明,过时效Al-Si-Mg-Er合金的热稳定性与析出相的平均尺寸密切相关。未引入自然时效时,Al-Si-Mg-Er合金中β''''''''相的平均尺寸小于Al-Si-Mg合金中β''''''''相的平均尺寸,且其尺寸分布更加集中。然而,当在人工时效之前引入自然时效时,Al-Si-Mg-Er合金中析出相的平均尺寸和分布均与Al-Si-Mg合金的类似。此时,两种合金具有相似的力学性能。此外,详细讨论Er对合金中析出相析出动力学的影响,来解释上述现象。
Abstract: The effect of Er on the microhardness and precipitation behavior of the heat-treated Al-Si-Mg alloy was investigated by microhardness tester and TEM. As a comparison, the influence of natural aging was also studied. It is shown that the thermal stability of the over-aged Al-Si-Mg-Er alloy is highly related to the average size of the precipitates. The average size of β'''''''' precipitates in Al-Si-Mg-Er alloy is smaller than that in Al-Si-Mg alloy, and the distribution is more localized under condition of without introducing natural aging. However, when natural aging is introduced before artificial aging, the Al-Si-Mg-Er alloy has similar average size and distribution of precipitates with the Al-Si-Mg alloy, resulting in similar mechanical properties. The effect of Er on the precipitation kinetics in the alloy was also discussed in detail to explain these phenomena.
Trans. Nonferrous Met. Soc. China 31(2021) 1-10
Yi HE, Hai-hui XI, Wen-quan MING, Qin-qin SHAO, Ruo-han SHEN, Yu-xiang LAI, Cui-lan WU, Jiang-hua CHEN
College of Materials Science and Engineering, Hunan University, Changsha 410082, China
Received 10 April 2020; accepted 20 July 2020
Abstract: The effect of Er on the microhardness and precipitation behavior of the heat-treated Al-Si-Mg alloy was investigated by microhardness tester and TEM. As a comparison, the influence of natural aging was also studied. It is shown that the thermal stability of the over-aged Al-Si-Mg-Er alloy is highly related to the average size of the precipitates. The average size of β'' precipitates in Al-Si-Mg-Er alloy is smaller than that in Al-Si-Mg alloy, and the distribution is more localized under condition of without introducing natural aging. However, when natural aging is introduced before artificial aging, the Al-Si-Mg-Er alloy has similar average size and distribution of precipitates with the Al-Si-Mg alloy, resulting in similar mechanical properties. The effect of Er on the precipitation kinetics in the alloy was also discussed in detail to explain these phenomena.
Key words: Al-Si-Mg alloy; Er; natural aging; precipitate; thermal stability
1 Introduction
Al-Si-Mg alloy has been widely used in many industrial fields, such as engine blocks and wheels, due to its good specific strength, excellent castability, low thermal expansion, high wear resistance and good corrosion resistance [1-6]. The as-cast alloy mainly consists of the primary α(Al) dendrites, irregular-shaped eutectic silicon phases and other intermetallic phases, such as Al5FeSi (β) and Al8Mg3Si6Fe (π). The coarse and irregular eutectic silicon phases could be the initial place of micro-cracks in the stress environment which may lead to poor ductility and low strength [2]. Therefore, chemical modification has been widely used to modify the Si morphology. For example, 10-4 order of Sr or Na can modify the eutectic Si phases from coarse flake-like structure to fine coral fibrous structure and improve both the strength and ductility of the alloy [7]. However, these modifiers have little effect on the α(Al) grain refinement.
To achieve better performance in modifying both the α(Al) grain size and the eutectic Si, in recent years, the rare earth (RE) elements, such as Sc [8,9], Er [10-14] La and Ce [15], have attracted a lot of attention. Although Sc was reported to have excellent effects on both grain refinement and eutectic Si modification, its application was extremely limited by the high cost. Er is an important modifier for Al-Si-Mg alloy because of its low cost and significant effectiveness. According to Ref. [10], 0.6 wt.% Er not only refined the grain size, but also modified the morphology of eutectic Si from coarse plate-like to fine fibers. It was also reported that the addition of Er to Al-Si-Mg-(Cu) alloys improved the yield stress (YS), ultimate tensile strength (UTS) and the ductility [10-14]. However, harmful porosity and Er-containing intermetallics formed during solidification increase obviously when Er addition is up to 0.4 wt.% [13]. So, the content of Er needs to be 0.2-0.3 wt.% to achieve the best mechanical properties.
Besides, the mechanical properties of the Al-Si-Mg alloy can be further improved by heat treatment through forming high-density nano-sized precipitates like 6000 series Al-Mg-Si alloy (mainly β'' phase or pre-β'' phase) [16]. A typical heat treatment procedure applied to the alloy is the T6 heat treatment, which includes solution treatment, quenching and artificial aging. During the solution treatment, the alloy can be homogenized and the irregular-shaped eutectic Si will fragment and spheroidize [17]. In the subsequent low-temperature aging process, high- density nano-sized precipitates, such as clusters, pre-β'' and β'' [18], are formed and the ultimate strength is enhanced. For Al-Si-Mg alloy without Er addition, the hardness and strength of the alloy decrease fast after the alloy reaches the peak-aged stage due to the coarsening of the precipitates [13]. Interestingly, it is found that the hardness of the Al-Si-Mg-Er alloy is more stable compared with Al-Si-Mg alloy at the over-aged stage [13]. Therefore, the resistance to over-aging effect or thermal stability of the Al-Si-Mg alloy can be improved with an appropriate Er content.
To understand the mechanism of better thermal stability, the microstructure of Al-Si-Mg-Er alloy has been observed by scanning electron microscope (SEM), XRD and so on. Some researchers proposed that the resistance to over-aging effect was associated with the Al3Er particles and the Er-containing intermetallics [13,14]. Nevertheless, this speculation should be more careful, since the quantity of Al3Er and Er-containing intermetallics formed during solidification is very small. In addition, these dispersoids should have the same strengthening contribution to the Al-Si-Mg-Er alloy whenever it is at the over-aged stage or early- aged stage, because they can hardly evolve in low- temperature (≤300 °C) thermal treatment [19,20]. However, it can be observed that at over-aged stage, the hardness increment between Al-Si-Mg-Er and Al-Si-Mg alloy is much higher than that at early-stage of aging [14]. In 6000 series Al alloy, researchers assumed that more precipitates formed in Er-containing alloys at peak-aged stage [21]. However, few evidences on the precipitate were provided correctly in the investigation.
In the present study, the microstructure and mechanical properties of Al-Si-Mg (A356) and Al-Si-Mg-Er (termed as A356-Er hereafter) alloys were investigated to uncover the influence of Er addition on the thermal stability during artificial aging treatment. Furthermore, the influences of natural aging (NA) prior to artificial aging on both alloys were also studied to confirm the proposed mechanism.
2 Experimental
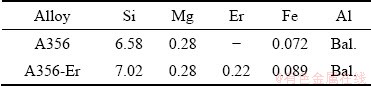
Two alloys with compositions of Al-7Si- 0.3Mg and Al-7Si-0.3Mg-0.2Er (wt.%) were cast using ZG-0.025 vacuum induction melting furnace and the detailed compositions are presented in Table 1. In the Al-Si-Mg-Er alloy, Er was added by Al-5.0wt.%Er master alloy.
Table 1 Chemical composition of alloys (wt.%)
Samples taken from the middle of the ingots with different alloy composition (A356 and A356-Er) were firstly solution-treated (SHT) at 550 °C for 5 h followed by water quenching to room temperature (RT). Subsequently, they were naturally aged (NA) at 20 °C for various time (0, 7 and 30 d) and then artificially aged (AA) at 180 °C in an oil bath for different durations.
The microhardness was measured in Vickers with a load of 0.2 kg for a dwell time of 15 s. Each hardness value in the present study was averaged by at least 5 indentations. The scanning electron microscopy (SEM) analysis was carried out with an FEI QUANTA 200 instrument. For SEM observation, the samples were further etched with solution containing 25 vol.% HNO3 and 75 vol.% methanol for 90 s after mechanically polishing in order to reveal the three-dimensional morphology of eutectic Si phases. The transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) observations were performed with an FEI Tecnai G2 F20S-Twin microscope operating at 200 kV. The TEM specimens were prepared firstly by mechanically polishing to a thin foil under 100 μm, and then punched into disks with 3 mm in diameter. After that, the disks were electro-polished in an electrolyte consisting of 25% HNO3 and 75% methanol at about -25 °C. All TEM observations were carried out along the direction of <001>Al.
3 Results and discussion
3.1 Microstructure of as-cast and solution- treated alloys
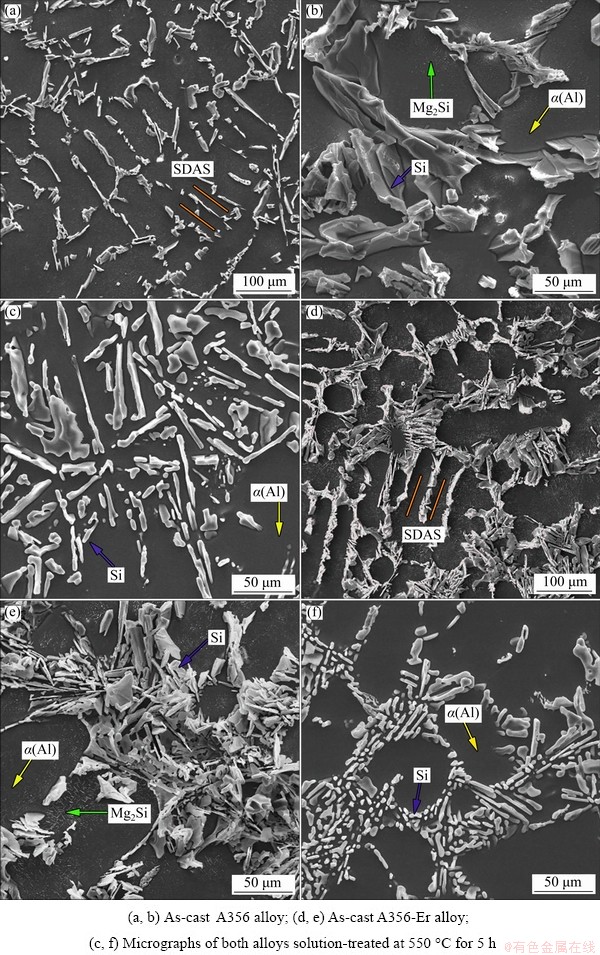
Fig. 1 SEM images of A356 (a-c) and A356-Er (d-f) alloys under as-cast and solution-treated conditions
Figure 1 shows the microstructure of both the A356 and A356-Er alloys under as-cast and solution-treated conditions. Figures 1(a) and (d) show low-magnification SEM images (secondary electron mode) of the as-cast alloys. Apparent dendrites can be seen in both alloys with secondary dendrite arm spacing (SDAS) being (45.9±7.7) μm for A356 alloy and (42.7±7.5) μm for A356-Er alloy. This confirms that both alloys have similar cast condition, which will make no difference in the hardness measurement and TEM observation in both alloys. The magnified images of the eutectic Si in A356 and A356-Er alloys are displayed in Figs. 1(b) and (e), respectively. In Fig. 1(b), the eutectic Si is coarse and plate-like. When Er element is added, the eutectic Si changes to coral-like structure and becomes thinner, as shown in Fig. 1(e). This is consistent with previous reports that Er addition can modify the morphology of eutectic Si phase [11,13]. In addition, small dispersoids marked by green arrows in α(Al) can be seen in Figs. 1(b) and (e). EDS analyses show that these dispersoids are composed of Si and Mg. Therefore, they can be identified as Mg2Si phases formed during solidification. After the solution treatment at 550 °C for 5 h, these dispersoids dissolve into the matrix for later precipitation and cannot be seen in Figs. 1(c) and (f). At the same time, the eutectic Si phases in both alloys fragment and spheroidize to some extent. By comparing Fig. 1(c) with Fig. 1(f), it can be seen that the spheroidization of eutectic Si in A356-Er alloy is faster than that in A356 alloy, since the eutectic Si in Fig. 1(f) is smaller and the aspect ratio is closer to 1.
3.2 Hardness responses of aged alloys
The evolution of microhardness for A356 and A356-Er alloys treated with various NA and AA time is shown in Fig. 2. Both alloys have similar initial hardness before aging, i.e. HV 54.5 for A356 alloy and HV 58.0 for A3356-Er alloy. With NA time extending, the hardness of both alloys increases gradually and reaches the peak value (HV ~72) after 3 d (Fig. 2(a)). Then, the hardness remains almost the same as NA treatment continues. The hardness increment can be attributed to nano-clusters formed during NA, of which the Mg/Si atomic ratio is approximately 0.6 [18]. After NA treatment for about 3 d, the strengthening clusters reach their maximum volume fraction and remain almost the same even though extending the NA time to 30 d. In the following experiment, 7-30 d NA treatment was applied before AA treatment at 180 °C. As a reference, AA treatment without NA was also conducted.
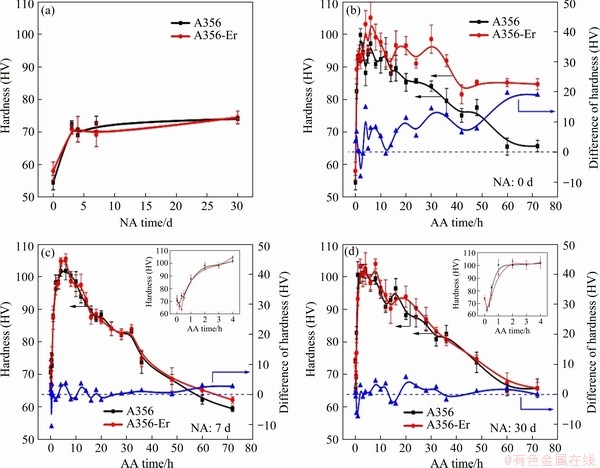
Fig. 2 Evolution of hardness during natural aging for A356 and A356-Er (a) and evolution of hardness during artificial aging at 180 °C after various NA time of 0 d (b), 7 d (c) and 30 d (d)
Figure 2(b) shows the hardness curves of both alloys aged at 180 °C without prior NA. It can be seen that the hardness of both alloys increases rapidly as AA begins and reaches the peak hardness at about 5 h. The peak hardness of A356-Er alloy is about HV 104.2, slightly higher than that of A356 alloy (HV 99.6). With aging time extending, the hardness of A356 alloy decreases immediately due to the over-aging effect [3,13]. The hardness of A356-Er alloy decreases as well, but is still much higher than that of A356, as shown by the red curve in Fig. 2(b). At aging time of 72 h, the hardness of A356 and A356-Er alloys is HV 65.7 and HV 84.6, respectively. This demonstrates that thermal stability of A356-Er alloy is much better than that of A356 alloy. The difference between the hardness of A356-Er and A356 is shown by the blue curve in Fig. 2(b). It is shown that the difference increases with extending the AA time. It is well known that the Al3Er dispersoids are very stable during thermal aging at temperature lower than 300 °C [19]. Therefore, the increment of hardness cannot be attributed to the stable Al3Er dispersoids. Since the precipitation of small particles happens during the thermal aging, it is reasonable to speculate that this increment of hardness is closely related to the precipitation process.
When prior NA for 7 or 30 d is applied, the hardness responses of both alloys become similar, as shown in Figs. 2(c) and (d), respectively. The inserts in Figs. 2(c) and (d) are magnifications of hardness curves of the first 4 h. It can be seen from the enlarged sub-figures in Figs. 2(c) and (d) that a slight decrease in hardness happens right after the AA begins, as compared with Fig. 2(b). This may be caused by the dissolving of clusters formed during natural aging [18,22]. Then, rapid hardness increase appears in both curves due to the precipitation of strengthening phases such as pre-β'' and β''. As artificial aging continues, the hardness of A356 keeps almost the same, as compared with black curves in Fig. 2(b). This is due to the similar precipitates volume fraction in A356 alloys with or without NA [18]. At the same time, the hardness of A356-Er alloy at over-aged stage decreases and has the same trend with that of A356 alloy, whenever the prior NA time is 7 or 30 d. The difference between hardness of A356-Er and A356 alloys remains unchanged and is nearly zero in these cases, as indicated by blue curves in Figs. 2(c) and (d). Therefore, it is interesting that the high thermal stability of A356-Er alloy at over-aged stage diminishes when natural aging is applied before artificial aging.
3.3 Microstructure of aged alloy by TEM observation
In the current study, it is found that natural aging inhibits the thermal stability of A356-Er alloy, while it has little influence on A356 alloy. However, the mechanism for the enhancement of thermal stability in A356-Er alloy without natural aging and the role of Er atoms in the precipitation during aging treatment are uncovered. To answer the questions, the precipitates should be carefully checked by TEM, since natural aging mainly influences the precipitation kinetics of Al-Si-Mg alloy during artificial aging [18,22]. In the following, the peak-aged and over-aged samples of both alloys without or with natural aging (7 d) were characterized by TEM, regarding the precipitate type and length. All TEM images were recorded with incident electron beam along the <001>Al direction because all precipitates in the alloy grow along this direction.
Figure 3 shows the bright-field TEM images and the length distribution of precipitates in both alloys without applying prior NA. The insert in each sub-figure is the representative HRTEM image of one precipitate in each sample. It can be seen that dense and fine needle-like precipitates are formed in both A356 and A356-Er alloys at peak-aged stage (Figs. 3(a) and (b)). By combining the HRTEM image and corresponding FFT, the lattice constants of the precipitate in Figs. 3(a) and (b) are measured to be a=1.51 nm, c=0.65 nm and β=106.4°, which are consistent with those of the β'' phase [23,24]. The length distributions of more than 200 precipitates were measured using high- magnification bright-field TEM images and are displayed in Fig. 3(c). It can be seen that the average length in A356-Er alloy is about 22.9 nm, smaller than that in A356 alloy. Besides, the length distribution of precipitates in A356-Er is more localized, indicating that the precipitates in A356-Er are more uniform.

Fig. 3 Bright-field TEM images of alloys peak-aged (a-c) and over-aged (d-f) at 180 °C
As artificial aging continues, precipitates grow longer, as shown in Figs. 3(d) and (e). HRTEM analysis demonstrates that all of these precipitates are still β''-type phases, although some precipitates are quite longer than others. In addition, many Si precipitates with diameter about 50 nm can also be seen at the over-aged stage in both alloys. Statistics of precipitate lengths (Fig. 3(f)) shows that the average length increases to 36.3 nm and 28.2 nm for A356 and A356-Er alloys, respectively. From Figs. 3(c) and (f), it is clear that the length of precipitates in A356-Er alloy is always smaller than that in A356 alloy during the thermal aging. Therefore, it is reasonable that the hardness of A356-Er is higher than that of A356 alloy (Fig. 2(b)).
For both alloys with prior NA applied, the precipitates were also characterized, as shown in Fig. 4. At the peak-aged stage (Figs. 4(a) and (b)), the precipitates are still β''-type phases in both alloys, which can also be confirmed by the inserted HRTEM images. While at the over-aged stage, besides the β''-type and Si phases, small portion of β' phases are also observed in both alloys. Typical HRTEM images of β'-type precipitates in A356 and A356-Er alloys and the corresponding FFT patterns are displayed in Figs. 4(c) and (f), respectively. Since β' is the later-stage precipitate during thermal treatment, its size is bigger than that of β'' precipitate, as shown in Figs. 4(c) and (f). Therefore, the abnormally long precipitates in Figs. 4(d) and (e) should be identified as β'. Note that the β'-type phase does not exist in the over-aged A356 and A356-Er alloys when prior natural aging is not applied (Figs. 3(d)-(e)).
Figure 5 displays the length distributions of precipitates in A356 and A356-Er alloys with prior NA treatment for 7 d. For the peak-aged A356 alloy, the average length of the precipitates is 26.8 nm, which is quite close to that in the peak-aged A356-Er alloy (28.3 nm as shown in Fig. 5(a)). With the AA time extending to 74 h, the β'' phases in both alloys were coarsened, as shown in Fig. 5(b). In this case, the average length of the precipitates in A356-Er alloy is 46.5 nm, similar with that in A356 alloy (49.6 nm). However, it should be noted that the fitted blue curve in Fig. 5(b) ignores the precipitates with length about 70 nm. Therefore, the actual length should be slightly higher than 46.5 nm. In addition, from the enlarged image in Fig. 5(b), it can be observed that there are much longer precipitates (>140 nm) in A356-Er alloys, while the longest length of precipitates in A356 alloy is about 140 nm. Therefore, under this circumstance, the precipitates should have similar strengthening contribution to the alloy, corresponding to similar hardness of A356 and A356-Er alloys (Fig. 2(c)).

Fig. 4 Bright-field TEM images of alloys at peak aging (a, b) and over aging (c, f) at 180 °C after NA for 7 d

Fig. 5 Size distributions of precipitates in both alloys with NA for 7 d
3.4 Effect of Er addition on β'' precipitate
In previous studies, the influence of Er addition on the thermal stability in A356 alloy was investigated [13]. In their opinion, the Al3Er particles and Er-rich coarse intermetallics formed during solidification should be responsible for the better thermal stability of A356-Er alloy. However, the Al3Er particles and the Er-rich coarse intermetallics formed during solidification are very stable at temperatures lower than 300 °C [14,19], so they can hardly evolve during NA or AA at 180 °C. Therefore, this explanation cannot interpret the phenomena in our study: firstly, the difference of hardness between A356 and A356-Er alloys varied with the aging time and secondly, the thermal stability of A356-Er alloy at over-aged stage was inhibited by introducing NA prior to AA (Fig. 2). In the present study, the β'' precipitates in A356-Er alloy were refined at both peak-aged stage and over-aged stage when prior NA was not applied. Therefore, it is reasonable to speculate that the influence of Er on precipitation of β'' phases may be the key reason for the mechanical property improvement.
Another important question is how Er addition influences the β'' precipitation in A356-Er alloy. According to Refs. [25,26], the nucleation rate of precipitates can be evaluated by the thermodynamic nucleation energy barrier (△G) given by
 (1)
(1)
where f is the shape factor of the nucleus, which is smaller for heterogeneous nucleation than that for homogeneous nucleation [25], γ is the interface Gibbs energy density between precipitate and matrix, and F is the driving force of precipitate formation.
When the alloy is solution-treated at 550 °C, a small amount of Er solute atoms (about 8×10-4 at.%) are uniformly distributed in Al matrix after quenching in water. The Al lattice around each Er atom is distorted due to the larger atom radius of Er (0.1734 nm) than that of Al atom (0.1432 nm). These distortions provide additional energy for the heterogeneous nucleation of Si-excess clusters that can evolve to β'' precipitates during aging. Therefore, the energy barrier △G is smaller than that in homogeneous nucleation due to the smaller parameter f in Eq. (1). The lower △G results in the increase of nucleation rate of β'' precipitates and the decrease in precipitate length (Fig. 3).

Fig. 6 Schematic illustration of precipitation scenario during AA for A356 and A356-Er alloys with various natural aging
To clearly show the precipitation scenario, a schematic illustration along the <001>Al direction is drawn, as shown in Fig. 6. For simplicity, the edge-on precipitates are ignored in this figure. When the A356 alloy is directly aged at 180 °C after quenching, the homogeneously nucleated clusters at the early-aged stage start to grow and transform into the β'' precipitates (Fig. 6(b)). Then, the volume fraction of precipitates reaches the maximum at the peak-aged stage. Besides, some clusters also exist at this stage [18]. As aging continues, the β'' precipitates grow longer (Fig. 6(c)), but keep the same structure. For the A356-Er alloy, the Er atoms can act as the heterogeneous nucleation sites for clusters (red dots in Fig. 6(d)). So the number of nucleation site in A356-Er alloy is higher than that in A356 alloy. In this case, the precipitates in A356-Er alloy are expected to be smaller than those in A356, since more precipitates are formed (Figs. 6(e) and (f)). This is consistent with the experiment results (Figs. 3(c) and (f)).
However, with prior NA applied, some NA clusters are formed in A356 alloy during NA and part of them can be the nucleation sites of β'' precipitates during AA [27,28], as shown by black circles in Fig. 6(g). The β'' precipitates from these clusters should be longer than those from the homogeneous nucleation sites (Fig. 6(h)), which results in a broader distribution of precipitate length compared with Fig. 6(b). In addition, the bigger β'' precipitates prefer to transforming into the β' phase to decrease the energy of the system (Fig. 4(c)). For the A356-Er alloy, apart from the ordinary NA clusters (black circles), the NA clusters with Er atoms (red circles in Fig. 6(j)) will also be formed during the long-term natural aging, and their size should be bigger than that of the NA clusters without Er atoms due to their lower energy barrier. Under this condition, these clusters grow faster and transform into much bigger β'' precipitates (red sticks in Fig. 6(k)) at peak-aged stage. These big precipitates further transform into the coarse β' precipitates to reduce the energy, and the distribution of precipitate length is broader, as shown in Fig. 6(l). This can be evidenced by much longer precipitates shown in the inserted image in Fig. 5(b). The proposed mechanism is reasonable since it is consistent with the experiment results.
4 Conclusions
(1) When directly aged at 180 °C, the A356-Er alloy has better thermal stability than A356 alloy without Er. This is due to the smaller length of β'' precipitates and more localized length distribution in A356-Er alloy.
(2) Natural aging has a negative effect on artificial aging at 180 °C in A356-Er alloy. TEM results demonstrate that when natural aging is applied before artificial aging, the A356-Er alloy has similar microstructure, including the average length and type of precipitate, with A356 alloy.
(3) The addition of Er to Al-Si-Mg alloy promotes the heterogeneous nucleation of β'' precipitates during artificial aging at 180 °C.
Acknowledgments
The authors are grateful for the financial supports from the National Key Research and Development Program of China (2016YFB0300801) and the National Natural Science Foundation of China (11904093, 51831004, 51671082, 51471067 and 11427806).
References
[1] ZHU Man, JIAN Zeng-yun, YANG Gen-cang, ZHOU Yao-he. Effects of T6 heat treatment on the microstructure, tensile properties, and fracture behavior of the modified A356 alloys [J]. Materials and Design, 2012, 36: 243-249.
[2] MAO Guo-ling, ZHU Cong-cong, WANG Shuai, YAN Han, GAO Wen-li. The role of yttrium modifying A357 alloy with sand casting [J]. Materials Science and Technology, 2019, 6: 1-7.
[3] LONG Hui-chi, CHEN Jiang-hua, LIU Chun-hui, LI Dian-zhong, LI Yi-yi. The negative effect of solution treatment on the age hardening of A356 alloy [J]. Materials Science and Engineering A, 2013, 566: 112-118.
[4] MAO Guo-ling, YAN Han, ZHU Cong-cong, WU Zhen, GAO Wen-li. The varied mechanisms of yttrium(Y) modifying a hypoeutectic Al-Si alloy under conditions of different cooling rates [J]. Journal of Alloys and Compounds, 2019, 806: 909-916.
[5] LI Hua-pei, TENG Jie, CHEN Gang. Wear mechanism for spray deposited Al-Si/SiCp composites under dry sliding condition [J]. Journal of Central South University, 2015, 22: 2875-2882.
[6] LI Qing-lin, XIA Tian-dong, LAN Ye-feng, LI Peng-fei, FAN Lu. Effects of rare earth Er addition on microstructure and mechanical properties of hypereutectic Al-20% Si alloy [J]. Journal of Alloys and Compounds, 2013, 588: 97-102.
[7] KUMARI S S S, PILLAI R M, PAI B C. Structure and properties of calcium and strontium treated Al-7Si-0.3Mg alloy: A comparison [J]. Journal of Alloys and Compounds, 2008, 460: 472-477.
[8] PANDEE P, GOURLAY C, BELYAKOV S, PATAKHAM U. AlSi2Sc2 intermetallic formation in Al-7Si-0.3Mg-xSc alloys and their effects on as-cast properties [J]. Journal of Alloys and Compounds, 2018, 731: 1159-1170.
[9] PRAMOD S L, RAVIKIRANA, RAO A K P, MURTY B S, RAJ C R. Effect of Sc addition and T6 aging treatment on the microstructure modification and mechanical properties of A356 alloy [J]. Materials Science and Engineering A, 2016, 674: 438-450.
[10] PANDEE P, PATAKHAM U, LIMMANEEVICHITR C. Microstructural evolution and mechanical properties of Al-7Si-0.3Mg alloys with erbium additions [J]. Journal of Alloys and Compounds, 2017, 728: 844-853.
[11] SHI Zhi-ming, WANG Qiang, ZHAO Ge, ZHANG Rui-ying. Effects of erbium modification on the microstructure and mechanical properties of A356 aluminum alloys [J]. Materials Science and Engineering A, 2015, 626: 102-107.
[12] HU Xiao-wu, JIANG Fu-gang, AI Fan-rong, YAN Hong. Effects of rare earth Er additions on microstructure development and mechanical properties of die-cast ADC12 aluminum alloy [J]. Journal of Alloys and Compounds, 2012, 538: 21-27.
[13] COLOMBO M, GARIBOLDI E, MORRI A. Er addition to Al-Si-Mg-based casting alloy: Effects on microstructure, room and high temperature mechanical properties [J]. Journal of Alloys and Compounds, 2017, 708: 1234-1244.
[14] GARIBOLDI E, COLOMBO M. Characterization of innovative Al-Si-Mg-based alloys for high temperature applications [J]. Key Engineering Materials, 2016, 710: 53-58.
[15] LIN Gao-yong, LI Kun, FENG Di, FENG Yong-ping, SONG Wei-yuan, XIAO Meng-qiong. Effects of La-Ce addition on microstructure and mechanical properties of Al-18Si-4Cu-0.5Mg alloy [J]. Transactions of Nonferrous Metals Society of China, 2019, 29: 1592-1600.
[16] CHOMSAENG N, HARUTA M, CHAIRUANGSRI T, KURATA H, ISODA S, SHIOJIRI M. HRTEM and ADF-STEM of precipitates at peak-ageing in cast A356 aluminium alloy [J]. Journal of Alloys and Compounds, 2010, 496: 478-487.
[17] SJOLANDER E, SEIFEDDINE S. Artificial ageing of Al-Si-Cu-Mg casting alloys [J]. Materials Science and Engineering A, 2010, 210: 1249-1259.
[18] SHA G, MOLLER H, STUMPF W E, XIA J H, GOVENDER G, RINGER S P. Solute nanostructures and their strengthening effects in Al-7Si-0.6Mg alloy F357 [J]. Acta Materialia, 2012, 60: 692-701.
[19] ZHANG Yi, GAO Kun-yuan, WEN Sheng-ping, HUANG Hui, NIE Zuo-ren, ZHOU De-jing. The study on the coarsening process and precipitation strengthening of Al3Er precipitate in Al-Er binary alloy [J]. Journal of Alloys and Compounds, 2014, 610: 27-34.
[20] NIE Zuo-ren, HUANG Hui, GAO Kun-yuan, LI Bo-long, WANG Wei, CHEN Zi-yong, RONG Li, WEN Sheng-ping, LI Hong-mei, ZUO Tie-yong. The effect of erbium on the properties and microstructure of Al alloys [J]. Materials Science Forum, 2012, 706-709: 329-334.
[21] ZHAO Qian, YUAN Xiao-guang, HUANG Hong-jun, ZHAO Peng. Precipitation kinetics for β″ phase of Al-Mg-Si-Zr-XEr alloys [J]. Rare Metal Materials and Engineering, 2016, 45: 2889-2894.
[22] MOLLER H, GOVENDER G, STUMPF W E. The natural and artificial aging response of semi-solid metal processed alloy A356 [J]. Solid State Phenomena, 2008, 141-143: 737-742.
[23] ZANDBERGEN H W, ANDERSEN S J, JANSEN J. Structure determination of Mg5Si6 particles in Al by dynamic electron diffraction studies [J]. Science, 1997, 277: 1221-1225.
[24] CHEN J H, COSTAN E, HUIS M A, XU Q, ZANDBERGEN H W. Atomic pillar-based nanoprecipitates strengthen AlMgSi alloys [J]. Science, 2006, 312: 416-419.
[25] KOZESCHNIK E, SVOBODA J, FRATZL P, FISCHER F D. Modelling of kinetics in multi-component multi-phase systems with spherical precipitates. II: Numerical solution and application [J]. Materials Science and Engineering A, 2004, 385: 157-165.
[26] LIU Chun-hui, LAI Yu-xiang, CHEN Jiang-hua, TAO Guan-hui, LIU Li-mei, MA Pei-pei, WU Cui-lan. Natural- aging-induced reversal of the precipitation pathways in an Al-Mg-Si alloy [J]. Scripta Materialia, 2016, 115: 150-154.
[27] MURAYAMA M, HONO K, SAGA M, KIKUCHI M. Atom probe studies on the early stages of precipitation in Al-Mg-Si alloys [J]. Materials Science and Engineering A, 1998, 250: 127-132.
[28] BIROL Y. Restoration of the bake hardening response in a naturally aged twin-roll cast AlMgSi automotive sheet [J]. Scripta Materialia, 2006, 54: 2003-2008.
何 忆,席海辉,明文全,邵秦秦,沈若涵,赖玉香,伍翠兰,陈江华
湖南大学 材料科学与工程学院,长沙 410082
摘 要:采用显微硬度计和透射电镜研究Er元素对热处理Al-Si-Mg合金显微硬度和析出行为的影响。作为对 照,同时研究人工时效前引入自然时效对合金硬度和析出行为的影响。研究结果表明,过时效Al-Si-Mg-Er合金的热稳定性与析出相的平均尺寸密切相关。未引入自然时效时,Al-Si-Mg-Er合金中β''相的平均尺寸小于Al-Si-Mg合金中β''相的平均尺寸,且其尺寸分布更加集中。然而,当在人工时效之前引入自然时效时,Al-Si-Mg-Er合金中析出相的平均尺寸和分布均与Al-Si-Mg合金的类似。此时,两种合金具有相似的力学性能。此外,详细讨论Er对合金中析出相析出动力学的影响,来解释上述现象。
关键词:Al-Si-Mg合金;Er;自然时效;析出相;热稳定性
(Edited by Wei-ping CHEN)
Corresponding author: Wen-quan MING; Tel: +86-731-88664009; E-mail: wqming@hnu.edu.cn
DOI: 10.1016/S1003-6326(20)65474-7
1003-6326/ 2021 The Nonferrous Metals Society of China. Published by Elsevier B.V. & Science Press
2021 The Nonferrous Metals Society of China. Published by Elsevier B.V. & Science Press

