
Preparation of Ti by direct electrochemical reduction of solid TiO2 and its reaction mechanism
NIE Xin-miao(聂新苗), DONG Ling-yan(董凌燕), BAI Chen-guang(白晨光),
CHEN Deng-fu(陈登福), QIU Gui-bao(邱贵宝)
College of Materials Science and Engineering, Chongqing University, Chongqing 400044, China
Received 10 April 2006; accepted 25 April 2006
Abstract: An electrochemical method for the direct reduction of solid TiO2 was introduced, in which the oxygen is ionized, dissolved in the molten salt and discharged at the anode, leaving pure titanium at the cathode. Titanium was prepared from TiO2 via molten salt electrolysis, and experimental process was studied in detail. The reductive products of TiO2 were analyzed through XRD, SEM, XPS and so on. The results indicate that titanium with high purity is obtained by the electrochemical reduction. Through thermogravimetric analysis of molten CaCl2, it is shown that the dehydration of CaCl2 has to be strictly controlled to eliminate hydrolysis. The cyclic voltammetry studies on the TiO2 cathode electrochemical system show that the reduction of TiO2 is conducted step by step, from exterior to interior and from high-valence to low-valence. The conclusions were also confirmed by study on thermodynamics analysis. The current efficiency can be improved by loading different voltages in different stages.
Key words: titanium; titanium dioxide; reduction; thermodynamics; electrochemistry
1 Introduction
Titanium is a kind of structural and functional material, which has many desirable properties such as lightmass, high strength and corrosion-resistance, and widely temperature adaptability[1]. It is the perfect material in metallurgy, navigation and chemical industries, biomedicine and so on. Titanium will be “the third metal” after Fe and Al, and the 21st century will be the century of titanium[2, 3]. But the extraction of titanium is difficult, as the chemical property of titanium at elevated temperature is active and it is easy to react with O, N, C, H and so on. Even more, the purity of titanium that industry required is high, so it is necessary to select an excellent process. Since 2000, the electrochemical reduction of titanium dioxide has drawn great attention from the metallurgical researchers in home and abroad. It is a kind of new metallurgical process, which is friendly to the environment and low energy consumption. At present, there are many new extraction processes of titanium, such as FFC[4], EMR[5], OS[6-9], of which FFC will be the most promising process. In this article, experimental method was improved based on FFC. Finally, titanium was prepared from TiO2 via molten salt electrolysis. Corresponding to the experiment, electrochemistry and thermodynamics were studied, which provided theoretical support to the subsequent experiment.
2 Experimental
2.1 Material
In the experiment, TiO2 (AP, 99.2%), which was mixed with appropriate binder, was used as cathode material. Cathode was prepared by a series of processes such as mixing, screen separation, pressing and sintering. Graphite rod (500 mm×20 mm×10 mm) was used as anode and CaCl2 (AP) was adopted as molten salt.
2.2 Experiment condition
Electrolysis experiment was performed in electric resistance furnace (coupled with silicon controlled apparatus and 2273 potentiostat), in which Ar was continuously purged (1.5 L/min). The experiment equipment is shown in Fig.1. Pre-electrolysis and electrolysis temperature were respectively 850 ℃ and 900 ℃, and pre-electrolysis and electrolysis potentials were respectively 3.1 V and 3.2 V.
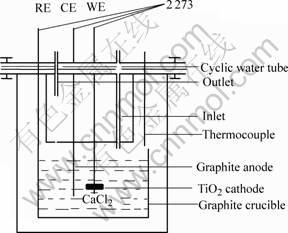
Fig. 1 Equipment sketch of experiment
2.3 Experiment procedure
The molten salt electrolysis experiment was performed in the equipment (Fig.1) containing molten CaCl2. Graphite rod was used as anode, and Ar was continuously purged into the equipment as protective gas. When the temperature reached 850 ℃, the electrodes were put into molten salt CaCl2. Pre-electrolysis for removing moisture and impurity from the molten salt was carried out at 2.8 V[10]. After pre-electrolysis of 2 h, electrolysis was carried out at 3.1 V[11] and the time of electrolysis was no less than 20 h. After finishing the electrolysis experiment, the electrodes were raised to avoid touching the molten salt CaCl2. With Ar continuously purging into the equipment, the reductive products of TiO2 were cooled in the equipment. After being cooled to room temperature, the reductive products of TiO2 were fetched and washed to detect.
2.4 Pretreatment of CaCl2
As water has great influence on the experiment, and molten CaCl2 has strong water absorbability, so the pretreatment of CaCl2 has to be carried out. Through pretreatment, a plenty of physical and crystal water was removed from the molten CaCl2[12]. Thermogravimetric analysis curves of CaCl2 was obtained (Fig.2).
Firstly, CaCl2 of 1mg was weighed; secondly, based on the experiment before, setting 100 ℃ for 20 min, and 300℃ for 20 min. According to the thermo- gravimetric analysis curve of CaCl2, some conclusions can be obtained as follows:
1) Two peaks at 244.3 ℃(removing crystal water) and 777.9 ℃(volatilization of CaCl2) are obvious, indicating that phase changes have taken place at the two temperatures.
2) The dehydration of CaCl2 at 100 ℃ is not sufficient, so the time in this stage has to be prolonged.
3) The dehydration of CaCl2 between 100-300 ℃ is rapid, the mass loss will not take place any more above 300 ℃.
4) When temperature reaches about 777.9 ℃, due to the volatilization of CaCl2, the mass loss accurs.
Through analysis above, when temperature reaches 300 ℃, the physical and crystal water of CaCl2 has been completely removed. In the light of quantity of CaCl2 (about 1.5 kg) used in the experiment, setting 100 ℃ for 2 h, and 300 ℃ for 1 h is executed.
2.5 Experimental results
The XRD pattern and SEM image of the electrolytic product are shown in Fig.3 and Fig.4 respectively. The XPS analyses conducted at spectrum 1 and 2 in Fig.4 are shown in Fig.5 and Fig.6, respectively.
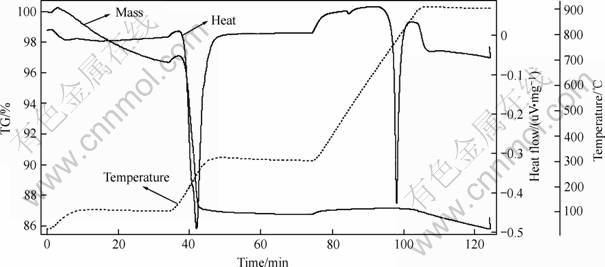
Fig. 2 Thermogravimetric analysis curves of CaCl2
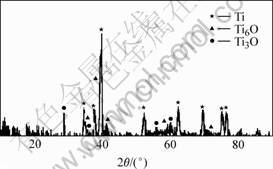
Fig. 3 XRD pattern of electrolytic product
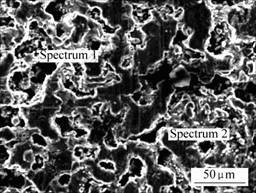
Fig. 4 SEM image of electrolytic product
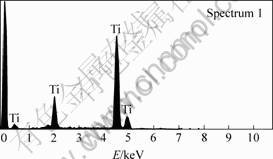
Fig.5 Analysis of XPS at spectrum 1 in Fig.4
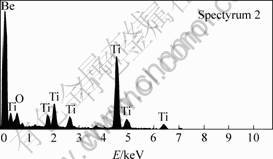
Fig.6 Analysis of XPS at spectrum 2 in Fig.4
3 Mechanism analysis
In order to explore the reaction mechanism of direct electro-reduction of titanium dioxide in molten calcium chloride, the electrochemical experiments were performed on Parstat 2273 Advanced Electrochemical System. It is shown that the reduction of TiO2 is conducted step by step, from exterior to interior and from high-valence to low-valence. Through a series of thermodynamics calculation, the decomposition potentials in different stages are achieved, which provide theoretical support to the subsequent experiment.
3.1 Electrochemical property of TiO2 cathode
3.1.1 Cyclic voltammogram of TiO2 electrode
A titanium dioxide bar was used as the working electrode. A graphite crucible was used as the pseudo-reference electrode. The counter electrode was a graphite rod. The cyclic voltammetry was performed on Parstat 2273 Advanced Electrochemical System. The cyclic voltammogram of TiO2 electrode is shown in Fig.7. When the scanning electric potential is in the range of -0.45 to -1.78 V, there exist two couples of redox peaks. Through analyzing the ions which exist in molten CaCl2, the other ions are impossible to redox in the field of electrochemical research, so it is apparent that two couples of redox peaks are in relation with TiO2.
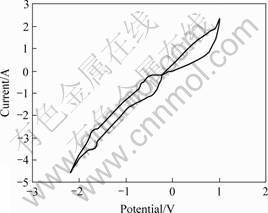
Fig.7 Cyclic voltammetric curves of TiO2 electrode in molten CaCl2 at 1 173 K
3.1.2 Chronoamperometry curve of TiO2 electrode at 1 173 K
The relationship between the current and the time at different step voltages at 1 173 K is shown in Fig.8. Fetch the current (which is at the same time but not at different cathode potentials in Fig.8) to draw the curve of I(t)—E (Fig.9). After the reaction reaches its steady state, which is characterized by a current plateau with a limiting current Id, the reaction current does not increase any further, even if the voltage is increased. Therefore the reaction rate of TiO2 is determined instead by the mass transfer or the diffusion. When a half-cell reaction is reversible and with soluble products, the number of electrons transferred in the half-cell reaction can be calculated from the following equation[13].
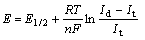 (1)
(1)
where E, E1/2, Id, It and n respectively represent the step voltage, the half-wave voltage, the limiting current, the current at time t, the number of electrons transferred in the half-cell reaction.
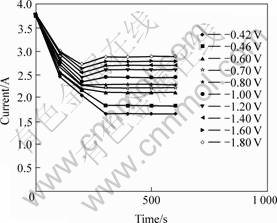
Fig. 8 Chronoamperomtery recorded on TiO2 electrode at 1 173 K
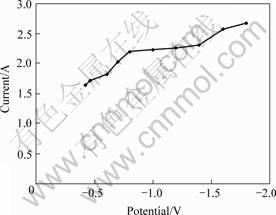
Fig. 9 Variation of I(t) with E for reduction of TiO2 in molten CaCl2 at 1 173 K
For the two steps of the reduction process of TiO2, the linear relationships between E and ln[(Id-It/It)] can be seen in Fig.10. The number of electrons transferred for the two steps was calculated to be 2 according to Eq.(1). The two electrons for both of the steps of the reduction process of TiO2 support the following reaction mechanism:
TiO2+2e=TiO+O2- (2)
TiO+2e=Ti+O2- (3)
3.2 Thermodynamics reaction mechanism
In order to confirm the cell voltage, firstly the decomposition of TiO2 should be confirmed. Aim at the cell reaction equation of TiO2:
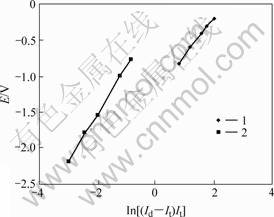
Fig. 10 Relationship between E and ln[(Id – It)/It] corresponding to Fig.9
TiO2(s)=Ti(s)+O2 (4)
TiO2+4e-=Ti+2O2- (Cathode) (5)
2O2-=O2+4e- (Anode) (6)
The theoretical decomposition potential can be obtained by thermodynamics calculation. The calculational principle is that electric energy (which satisfies compound decomposition) is equal to the forming Gibbs free energy, but the symbol is contrary[14]. The formula can be expressed as
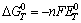 (7)
(7)
where 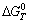 , n, F,
, n, F,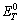 respectively represent the standard Gibbs free energy at constant voltage, the number of electrons transferred in the cell reaction, the Faraday constant and the theoretical decomposition potential at standard state. Based on the definition of Gibbs free energy (G=H-TS),
respectively represent the standard Gibbs free energy at constant voltage, the number of electrons transferred in the cell reaction, the Faraday constant and the theoretical decomposition potential at standard state. Based on the definition of Gibbs free energy (G=H-TS),
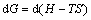 (8)
(8)
 (9)
(9)
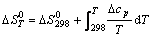 (10)
(10)
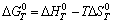 (11)
(11)
 (12)
(12)
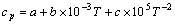 (13)
(13)
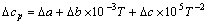 (14)
(14)
The thermodynamics data and the decomposition voltage are listed in Table 1 [15] and Table 2.
Table 1 Thermodynamics data of species
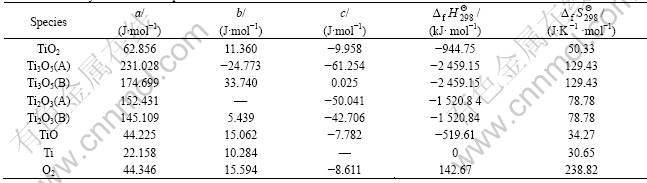
Table 2 Theoretical decomposition voltage of reaction formula
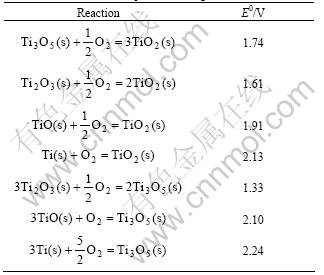
The theoretical decomposition voltage of TiO2 directly electrolyzing is high, which is 2.13V. But the theoretical decomposition voltages of TiO2 electrolyzing step by step are all below 2.13. So the direct reduction of TiO2 is conducted step by step. Loading different voltages in different stages can improve the current efficiency. As the thermodynamics data of low-valence titanium oxide has not been achieved, the research of reduction course step by step will go further.
4 Conclusions
1) Titanium of high purity was achieved in the experiment.
2) The dehydration of CaCl2 has to be strictly controlled.
3) By cyclic voltammetry study of the TiO2 cathode electrochemical system, it is shown that the reduction of TiO2 is conducted step by step.
4) Through thermodynamics calculation, the theoretical decomposition voltages of TiO2 electrolyzing step by step are obtained. The result shows that the theoretical decomposition voltage of TiO2 directly electrolyzing is high, but that of TiO2 electrolyzing step by step are all below the value. So the direct reduction of TiO2 is conducted step by step. Loading different voltages in different stages can improve the current efficiency.
References
[1] MO Wei, DENG Guo-zhu, LUO Fang-cheng. Titanium Metallurgy[M]. Beijing: Metallurgical Industry Press, 1998, 8-41, 281-332. (in Chinese)
[2] DENG Guo-zhu. Discussion of the achievement and the developing trend in titanium metallurgy[J].Chinese Journal of Rare Metals, 2002, 26(5): 391-396. (in Chinese)
[3] SUN Kang.Titanium Extraction[M]. Beijing: Metallurgical Industry Press, 2001, 49. (in Chinese)
[4] Fray D J. Electrochemical Processing using slags, fluxes and salts[A].VII International Conference on Molten Slags Fluxes and Salts[C]. 2004. 6-12.
[5] Park II, Abiko T, Okabe T H. Production of titanium powder directly from TiO2 in CaCl2 through an electronically mediated reaction[J]. Journal of Physics and Chemistry of Solids, 2005, 66: 410-413.
[6] Suzuki R O. Calciothermic reduction of TiO2 and in situ electrolysis of CaO in the molten CaCl2[J]. Journal of Physics and Chemistry of Solids, 2005, 66: 461-465.
[7] One K, Suzzuki R O. Calciothermic reduction of TiO2 [J]. Metall Mater, Material Jpn, 2002, 1: 28-31
[8] One K, Suzuki R O, Mem J O M. Calciothermic reduction of TiO2 [J]. Min Met Mater Soc, 2002, 54(2): 59-61.
[9] Suzuki R O, Ono K. Reaction mechanism of direct electro-reduction of titanium dioxide in molten calcium chloride [A]. Teste to Hagane. Proceedings of the 13th International Symposium on Molten Salt[C]. The Electrochemical Society, Penningston, NJ, 2002, 810-82.
[10] George Z, Chen and Derek J Fray. Volta metric Studies of the Oxygen-Titanium Binary System in Molten Calcium Chloride[J]. Journal of The Electrochemical Society, 2002, 149(11): 455-467.
[11] Fray D J. Emerging molten salt technologies for metals production[J]. JOM, 2001, 52(10): 26-31.
[12] Chen G Z, Fray D J, Farthing T W. Direct electrochemical reduction of titanium dioxide to titanium molten Calcium Chloride[J]. Nature, 2000, 407: 361-364.
[13] Guo J J, Shu Y Q. Thermodynamic and Dynamic Analysis of Titanium Alloy ISM Smelting Process[M]. Harbin: Harbin Institute of Technology Press, 1998. 2.
[14] WEI Qing-cheng. Metallurgy Thermodynamics. Chongqing: Chongqing University Press, 1996. (in Chinese)
[15] LIANG Ying-jiao, CHE Yin-chang. Data Manual of Mineral Thermodynamics[M]. Shenyang: Northeastern University Press, 1994.
(Edited by YUAN Sai-qian)
Foundation item: Project (2003AA33X210) supported by the Hi-Tech Research and Development Program of China
Corresponding author: NIE Xin-miao; Tel: +86-23-60891834; E-mail: nxm198198105@126.com