
Electrocatalytic properties of Ni-S-Co coating electrode for
hydrogen evolution in alkaline medium
YUAN Tie-chui(袁铁锤), LI Rui-di(李瑞迪), ZHOU Ke-chao(周科朝)
State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 26 September 2006; accepted 25 October 2006
Abstract: Amorphous Ni-S-Co alloy was prepared by means of chemical electro-deposition method on the foam nickel matrix. The surface morphology and microstructure of Ni-S-Co coatings were studied using SEM and XRD, and the electrochemical properties were tested by electrochemical methods. The results show that the coating has amorphous structure and the particles of the surface are fine with large specific surface area. The Ni-S-Co alloy is more active with lower potential for hydrogen evolution, higher exchange current density and lower activation energy compared with Ni and Ni-S electrode. Its hydrogen evolution reaction(HER) is enhanced, the size of particles of surface decreases and the surface area increases after being activated by KOH alkaline solution.
Key words: Ni-S-Co; amorphous alloy; electro-deposition; hydrogen evolution reaction
1 Introduction
As a part of the renewable energy cycle for sustainable systems, developing new methods and improving conventional technology for the electrolytic production of hydrogen, were getting continuous considerations. A principle focus of modern research in electrocatalysis is to discover electrode materials that exhibit desirable electrochemical stability and show improved electroactivity toward typical electrochemical reactions. It is also preferable that these materials are not expensive and are abundant. Electrocatalytic hydrogen evolution on various electrode materials and from various electrolyte solutions is one of the most frequently studied electrode reactions[1-4].
Electrolytic water splitting has gained importance in recent years because of economic production without adverse environmental impact. For water electrolysis becoming a more competitive and efficient process, the energy loss has to be minimized and the equipment cost has to be lowered. Therefore, the practical cell voltage should be reduced as far as possible in order to elevate the cost benefit of electric energy, since this is the main cost of the hydrogen production[5-7].
The electrocatalytic activity for the hydrogen evolution reaction(HER), and the bonding effectiveness of transition metals (such as Ni and Co) and their intermetallics are in close correlation. They are a periodic function of the atomic number within three long transition metal periods[8]. In this study, the Ni-S-Co electrode materials were prepared by adding CoSO4·7H2O into Ni-S electrolyte[9]. It has been found that Ni-S-Co electrode materials are a good electrocatalyst for the hydrogen evolution reaction(HER) in 30% KOH (mass fraction) aqueous solutions because of their optimum conductivity[10-12].
2 Experimental
2.1 Preparation of Ni-S-Co coating electrode
The preparation of the electrode, nickel flake (dimension: 15 mm×15 mm) was used as anode, foam nickel was used as cathode, and the concentrations of the nickel flake and the foam nickel are over 99%. In Ni-S-Co coatings deposition, a modified Watts bath was used which contains 120 g/L NiSO4·7H2O, 20 g/L CoSO4·7H2O, 50 g/L H3BO3, 30 g/L NaCl. The electrodepositing conditions are as follows: current density of 30 mA/cm, pH value of 4, temperature of 45 ℃, electrodepositing time of 60 min. After electro- deposition, the samples were cleaned, dried and leached in 30% KOH alkaline solution for 1-10 min.
2.2 Components and composition of Ni-S-Co deposits
The nickel, sulfur and cobalt contents were determined by EDX measurement. XRD (D/MAX-RB) and SEM (KYKY-2800) measurements were performed to determine the structures and morphologies of Ni-S-Co alloys.
2.3 Electrochemical measurements
The electrochemical properties were determined using a CHI660b (USA) potentiastat/galvanostat. Ni-S- Co alloys were used as the working electrodes. An Hg/Hg2Cl2 electrode was used as the reference electrode, and a thick Pt thread was employed as the counter electrode. A Luggin capillary was used to minimize the variations due to iR drop in the electrolytes. The galvanostatic experiments were carried out at different temperatures and 30% KOH solutions.
3 Results and discussion
3.1 Surface structure of Ni-S-Co coating electrode
Fig.1 shows the micrographs of amorphous Ni-S-Co alloys deposited on foam nickel substrate. It can be seen that the substrate has multilayer structure and is enrich of pores with the diameter of 0.3-0.4 mm. Compared with other electrodes, the multilayer structure has larger surface area and smaller particle size, which can improve hydrogen evolution reaction(HER) performance dramatically. EDX measurement shows that the content of Ni, S, Co are of 78.47%, 19.34%, 2.19%, respectively.

Fig.1 SEM Micrographs of Ni-S-Co amorphous coating
It is shown that the size of the hole on nickel matrix are 0.4-0.5 mm, the deposited particles are 3-5 ?m and the electrode coatings are made up of Ni-S-Co. This structure has larger specific surface area and better HER activity. Fig.2 shows XRD pattern of Ni-S-Co coating. Obviously, only the peaks of the base nickel phase can be observed, which indicates that the structure of the coating is amorphous.

Fig.2 XRD pattern of Ni-S-Co coating
3.2 Comparison of amorphous Ni-S-Co alloy elec- trode with other electrodes
Fig.3 shows the polarization curves of amorphous Ni-S-Co alloy, Ni-S alloy and nickel metal in a 30% (mass fraction) NaOH solution at room temperature. It can be seen that the HER activity of amorphous Ni-S-Co alloy is the highest among the three electrodes. The lower the HER overpotential, the higher the HER activity is under the same conditions. The HER overpotential of amorphous Ni-S-Co alloy is 100 mV and 400 mV lower than those of amorphous Ni-S alloy and nickel metal at current density of 120 mA/cm2, respectively. It shows that adding cobalt element in Ni-S alloy can obviously improve HER activity of the electrode.
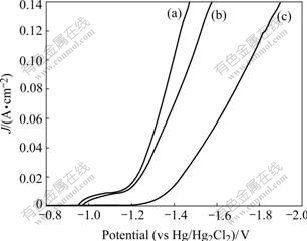
Fig.3 Polarization curves of various electrodes at room temperature (24 ℃): (a) Ni-S-Co; (b) Ni-S; (c) Ni
Kinetic parameters of three electrodes are listed in Table 1. It shows that the exchange current density(J0) increases with the temperature, which means that the temperature obviously influences HER overpotential. Increasing the electrolysis temperature can decrease HER overpotential. According to Arrhenius law, the relationship among current density, surface activity energy and temperature is as follows: lg J0=lg(Fkc)-?GΘ/ (2.3RT).
Table 1 Kinetic parameters of three electrodes for HER
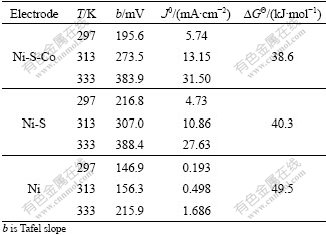
The value of lg J0 at the different temperature can be obtained by Farady curves. Fig.4 shows the relationship between exchange current density and T -1. The surface activity energy can be calculated from Fig.4, that is 49.5 kJ/mol for nickel electrode, 40.3 kJ/mol for Ni-S amorphous electrode and 38.6 kJ/mol for amorphous Ni-S-Co electrode. The surface activity energy(ΔGΘ) of amorphous Ni-S-Co electrode is much lower than that of other two electrodes, which indicates that its HER activity is higher than those of Ni-S alloy and nickel metal electrode.

Fig.4 Relationship between exchange current density and T -1
The high electrocatalytic activity can be explained by Brewer’s high temperature thermodynamics[13], which suggests that the intermetallic phases with very strong bonds, were made by bonding hypo- with hyper-d-electronic transition metals, where in accordance with the generalized Lewis acid-base reaction, there is a transfer of electrons from the orbital with paired d-electrons to the empty or half-filled d-orbital. It has been shown that more exposed d-orbital in the region from 3d to 5d correspond to the stronger cohesive and therefore weaker adsorptive bonding, resulting in higher electrocatalytic activity for the HER.
In spite of the fact that the electrocatalytic activity is in close correlation with the electronic configuration of individual metals and their intermetallics, some of the high electrocatalytic activities could additionally be explained on the basis of three-dimensional hydridic features of composite electrocatalysts, and the ability to absorb certain amount of hydrogen[14].
3.3 Influences of activation on HER properties of amorphous Ni-S-Co electrode
Fig.5 shows the HER polarization curves of amorphous Ni-S-Co electrode before and after KOH solution activation. It can be seen that HER properties are improved after KOH solution leaching treatment, the HER potential decreases by 10 mV at current density of 100 mA/cm2 after activation.
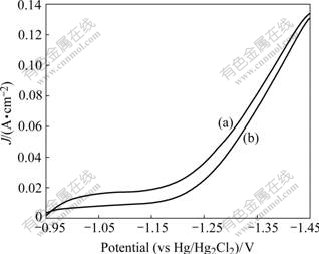
Fig.5 Influence of activation on HER properties of amorphous Ni-S-Co electrode: (a) After activation; (b) Before activation
Fig.6 shows the micrographs of amorphous Ni-S-Co coating after activation. It is evident that the increase of the surface area is due to the dispersed distribution of the small particles on the coating surface, thus improving the HER properties.

Fig.6 Micrograph of Ni-S-Co amorphous coating after activation
4 Conclusions
1) The structure of Ni-S-Co deposit is amorphous. The size of the particles on surface area become smaller after KOH solution leaching activation, so the catalytic properties of HER are improved. The HER potential decreases by 10 mV at current density of 100 mA/cm after activation.
2) Amorphous Ni-S-Co alloy electrode shows better HER properties. The HER overpotential of amorphous Ni-S-Co alloy was 100 mV and 400 mV lower than those of amorphous Ni-S alloy and nickel metal at current density of 120 mA/cm2, respectively. Also, the surface activity energy is calculated from experiment, that is, 49.5 kJ/mol for nickel electrode, 40.3 kJ/mol for Ni-S amorphous electrode and 38.6 kJ/mol for amorphous Ni-S-Co electrode. The amorphous Ni-S-Co electrode owns the lowest surface activity energy among these electrodes.
3) The elements co-operation improves the HER properties of Amorphous Ni-S-Co alloy electrode due to adding cobalt element.
References
[1] HAN Qing, LIU Kui-ren, CHEN Jian-she, WEI Xu-jun. A study on the electrodeposited Ni-S alloys as hydrogen evolution reaction cathodes [J]. International Journal of Hydrogen Energy, 2003, 28(11): 1207-1212.
[2] HINE F, YSUDA M, WATANABE M. Studies of the nickel-sulphur electrodeposited cathode [J]. Denki Kagaku, 1979, 47(2): 400-404.
[3] GONSLEZ E R, AVACA L A, TREMILIOSI-FILHO G. Hydrogen evolution reaction on Ni-S electrodes in alkaline solutions [J]. Int J Hydrogen Energy, 1994, 19(1): 15-19.
[4] PASEKA I. Sorption of hydrogen and kinetics of hydrogen evolution on amorphous Ni-Sx electrodes [J]. Electrochimica Acta, 1993, 38(16): 2449-2454.
[5] LI Ning, DING Da-yong, LI De-yu. Electrocatalytic behavior of amorphous Ni-Co-P coating for hydrogen evolution in alkaline medium [J]. Journal of Harbin Institute of Technology, 2005, 37(9): 1185-1188.
[6] KIRK D W, THORPE S J, SUZUKI H. Ni-base amorphous alloys as electro catalysts for alkaline water electrolysis [J]. Int J hydrogen Energy, 1997, 22(5): 493-500. (in Chinese)
[7] TRYGVE B. Hydrogen evolution on NiPX alloys: The influence of sorbed hydrogen [J]. Int J Hydrogen Energy, 2001, 26: 1193-1198.
[8] MARCETA KANINSKI M P, MAKSIC A D, STOJIC D L, MILJANIC S S. Ionic activators in the electrolytic production of hydrogen-cost reduction-analysis of the cathode [J]. Journal of Power Sources, 2004, 131: 107-111.
[9] LIU Fang, HE Han-wei, ZHOU Ke-chao, YUAN Tie-chui. Dependences of sulfur content during electro-deposition of Ni-S alloys [J]. Materials Science and Engineering of Powder Metallurgy, 2005, 10(1): 60-64. (in Chinese)
[10] HAN Qing, LIU Kui-ren, CHEN Jian-she, WEI Xu-jun. Hydrogen evolution reaction on amorphous Ni-S-Co alloy in alkaline medium [J]. International Journal of Hydrogen Energy, 2003, 28(12): 1345-1352.
[11] DU Min, GAO Rong-jie. The mechanism of hydrogen evolution reaction on amorphous Ni-S alloy coating [J]. Journal of Ocean University of Qingdao, 2003, 33(6): 961-968. (in Chinese)
[12] JAKSIC J M, RISTIC N M, KRSTAJIC N V. Electro catalysis for hydrogen electrode reactions in the light of Fermi dynamics and structural bonding factors (I): Individual electro catalytic properties of transition metals [J]. Int J Hydrogen Energy, 1998, 23(12): 1121- 1156.
[13] BREWER L. High-strength materials [M]. New York: Wiley, 1965: 12-103.
[14] LI X G, CHIBA A, TAKAHASHI S, AOKI K, MASUMOTO T. Changes in magnetic properties of C15 laves compound RCo2 due to hydrogenation [J]. Intermetallics, 1999, 7: 207-211.
Foundation item: Project(2003AA305980) supported by the National High-Tech Research and Development Program of China
Corresponding author: ZHOU Ke-chao; Tel: +86-731-8836264; E-mail: zhoukechao@mail.csu.edu.cn
(Edited by HE Xue-feng)