DOI: 10.11817/j.issn.1672-7207.2015.01.003
绿泥石与黄铁矿的异相凝聚机理
冯博1,冯其明2,卢毅屏2
(1. 江西理工大学 资源与环境工程学院,江西 赣州,341000;
(2. 中南大学 资源加工与生物工程学院,湖南 长沙,410083)
摘要:通过沉降实验、Zeta 电位测试、电子显微镜观测、溶解试验和溶液化学计算,研究绿泥石和黄铁矿的异相凝聚现象,并对其机理进行分析。研究结果表明:黄铁矿在实验所研究的pH范围内荷负电,未检测到等电点,绿泥石等电点pH约为4.5;当pH大于4.5时,绿泥石和黄铁矿表面均荷负电,颗粒间静电作用能为相互排斥,二者不会发生异相凝聚现象。由于矿浆中氧存在,调浆过程中黄铁矿表面的铁氧化溶出,溶出的铁离子在pH大于4.5时主要以羟基铁和氢氧化铁形式存在,羟基铁和氢氧化铁荷正电,吸附在绿泥石表面,使绿泥石的Zeta电位发生改变,氧化也使黄铁矿的Zeta电位发生变化,从而使绿泥石与黄铁矿表面电性相反,由于静电吸引作用而发生异相凝聚。
关键词:绿泥石;黄铁矿;氧化;电位;凝聚
中图分类号:TD952 文献标志码:A 文章编号:1672-7207(2015)01-0014-06
Mechanism of hetero-aggregation of chlorite and pyrite
FENG Bo1, FENG Qiming2, LU Yiping2
(1. School of Resource and Environment Engineering, Jiangxi University of Science and Technology,
Ganzhou 341000, China;
2. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract: The mechanism of hetero-aggregation of chlorite and pyrite was investigated through sedimentation tests, Zeta potential measurements, dissolution experiments, optical microscope photos and calculations of solution chemistry. The results show that the pyrite is negatively charged in the experimental pH range and thus its isoelectric point (IEP) is not determined. The IEP of chlorite is about pH 4.5. When pH is above 4.5, both chlorite and pyrite are negatively charged, and therefore the electrostatic energy among particles is mutually repulsive and the hetero-aggregation never occurs. With the existence of oxygen in solution, the iron ions on the surface of pyrite are oxidized and dissolved in the process of pulp conditioning. The dissolved iron ions exist mainly in the form of iron hydroxyl and iron hydroxide, both of which are positively charged, bringing about the adsorption on chlorite surface. Consequently, the Zeta potentials of both pyrite and chlorite are changed to a different extent, causing their opposite electrical behavior and inter-attraction between them due to electrostatic interaction. So the hetero-aggregation occurs.
Key words: chlorite; pyrite; oxidation; potential; hetero-aggregation
中国金川镍矿是以硫化铜镍矿石为主的特大型多金属共生矿,是当前我国镍的主要生产基地,但矿石中有用硫化矿物与含镁硅酸盐脉石矿物的浮选分离比较困难,而后续冶炼工艺要求精矿中MgO的质量分数低于6.8%[1],因此,在浮选过程中,对含镁硅酸盐矿物的抑制显得十分重要。金川硫化铜镍矿中含镁脉石主要是蛇纹石、绿泥石和滑石等含镁硅酸盐矿物[2]。许多研究者研究了含镁脉石进入精矿的原因。Fornasiero等[3]研究表明:当pH为7~10时,Cu(Ⅱ)和Ni(Ⅱ)可以活化石英、蛇纹石和绿泥石等硅酸盐矿物的黄药浮选。Pietrobon等[4-5]经研究发现泡沫夹带是微细粒蛇纹石浮选进入精矿的重要原因。还有研究者[6-7]发现有用矿物与脉石矿物之间的表面电性差异将会使矿物颗粒之间产生“异相凝聚”作用,使脉石吸附在有用矿物表面,形成矿泥罩盖。矿泥罩盖不仅会使矿泥附着在硫化矿表面上浮进入精矿,还会阻止硫化矿与气泡接触,降低硫化铜镍矿浮选速率和回收率[8]。因此,研究脉石矿泥和硫化矿之间的异相凝聚具有十分重要的意义。多年来,人们认为矿物颗粒之间由于电性相反所产生的静电吸引作用是形成矿泥罩盖的主要原因[9-11]。如荷负电的氢氧化铁罩盖在方铅矿表面[12]、荷正电的蛇纹石罩盖在镍黄铁矿表面[13]。许多研究者认为荷相同电荷的矿物之间不会发生异相凝聚,如Edwards等[13]提出绿泥石与镍黄铁矿均荷负电,二者不会发生异相凝聚。本文作者通过沉降实验、光学显微镜观测、Zeta 电位测试、溶解试验和溶液化学计算,对表面电荷相同的绿泥石、黄铁矿之间的异相凝聚现象进行研究,并对其作用机理进行分析。
1 实验
1.1 矿样与试剂
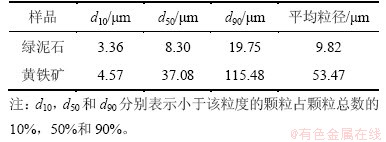
绿泥石矿样取自辽宁海城绿泥石矿;黄铁矿试样取自广东云浮。实验用纯矿物制备方法为:人工选取块矿,经锤碎手选后用瓷球磨、搅拌磨磨细。以粒度小于19 μm的绿泥石和小于115 μm的黄铁矿作为凝聚分散实验试样,表1所示为各矿物样品的粒度组成。实验用盐酸、氢氧化钠均为分析纯,实验用水为一次蒸馏水。
1.2 实验方法
1.2.1 沉降实验
本文采用矿浆的浊度表征矿粒的分散性,浊度越大,表明其分散性越好。沉降实验在100 mL沉降量筒中进行,以绿泥石质量浓度为0.5 g/L,按实验条件调浆后,沉降3 min,抽取上部25 mL悬浮液,用散射光浊度仪WGZ-3测定浊度(浊度单位为NTU,1 mg/L SiO2悬浊液的浊度为1 NTU)。人工混合矿沉降实验方法同单矿物实验,其中每次实验的绿泥石用量为0.05 g,黄铁矿为1 g。
表1 矿物样品的粒度组成
Table 1 Size composition of samples
1.2.2 Zeta电位测试
将绿泥石和黄铁矿纯矿物细磨至粒径小于2 μm,用高精度天平称取30 mg矿物,把矿样放入100 mL的烧杯中,加入50 mL蒸馏水,加入实验药剂并调节pH。采用磁力搅拌器搅拌5 min,然后采用Coulter Delsa440sx Zeta电位分析仪进行Zeta电位测量。每个点均测3次后取平均值。黄铁矿电位测定前需用超声处理,氧化黄铁矿电位测定前不用超声处理。实验所用支持电解质为1 mmol/L的KNO3溶液。
1.2.3 光学显微镜观测
按沉降试验条件进行绿泥石和黄铁矿人工混合矿的调浆,在矿浆搅拌状态下用针管抽取少量矿浆滴在载玻片上,将载玻片置于光学显微镜下观察矿物的分散状态,利用与光学显微镜相连的摄像头获取照片。
1.2.4 ICP测试
黄铁矿在一定的矿浆浓度及pH条件下,搅拌调浆后,离心分离,得到上清液,使用PS-6真空型等离子体原子发射光谱仪ICP-AES分析上清液中Fe的含量。
2 结果与讨论
2.1 绿泥石与黄铁矿表面电性及相互作用
图1所示为绿泥石与黄铁矿的Zeta电位随pH的变化。由图1可知:随着pH升高,绿泥石Zeta电位降低并由正变为负;当pH=4.5时,ζ=0 mV,故绿泥石的等电点pH为4.5,这与其他研究者得到的结论相符[14]。黄铁矿表面在所研究的pH范围内荷负电,未测试到等电点;当pH>4.5时,绿泥石和黄铁矿表面均荷负电,二者之间存在静电排斥作用。

图1 矿物Zeta电位与pH的关系
Fig. 1 Relationship between Zeta potential of minerals and pH
本实验采用光浊度法来表征矿粒在水中的分散性,浊度越大,表明分散越好,浊度减小表明矿物颗粒间发生凝聚。由于试验选用的黄铁矿粒度较粗,在实验条件和pH范围内,黄铁矿易沉降,其初始质量浓度为10 g/L的矿浆,浊度仅为35 NTU。因此,绿泥石单矿物的浊度可以用来表征混合矿的理论浊度,混合矿浆浊度的变化反映绿泥石与黄铁矿间的异相凝聚/分散现象。图2所示为绿泥石和黄铁矿人工混合矿矿浆浊度与pH的关系(初始绿泥石质量浓度为0.5 g/L,黄铁矿质量浓度为10 g/L)。由图2可知:绿泥石和黄铁矿人工混合矿的实际浊度远远小于理论浊度,表明在实验所研究的pH范围内,绿泥石与黄铁矿发生了异相凝聚,这与矿物表面电性结果不相符。

图2 人工混合矿浊度与pH的关系
Fig. 2 Relationship between turbidity of artificial minerals and pH
为了进一步证实绿泥石与黄铁矿发生了异相凝聚,对黄铁矿和绿泥石混合调浆后的凝聚分散状态进行了显微镜下观察。将黄铁矿同绿泥石按照质量比为2:1的比例混合,在pH=9及pH=3的条件下进行调浆,进行显微镜下观察,结果如图3所示。图中黑色大颗粒为黄铁矿而小颗粒为绿泥石。由图3可知:在2种pH条件下,绿泥石与黄铁矿均发生了显著的异相凝聚现象。
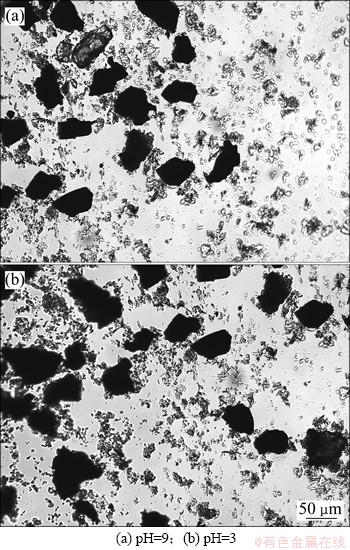
图3 绿泥石与黄铁矿分散聚集状态
Fig. 3 Dispersion and aggregation state of chlorite and pyrite
2.2 黄铁矿表面氧化对矿物电位及相互作用的影响
在磨矿和调浆过程中,由于矿浆中氧气的存在,黄铁矿表面容易氧化,使铁离子从黄铁矿表面溶解下来[15-18]。黄铁矿和氧气反应、溶解的过程可以用下列方程表示:
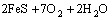 →
→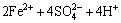 (1)
(1)
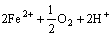 →
→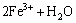 (2)
(2)
方程(1)和(2)合并可得
 →
→ (3)
(3)
由方程(1)~(3)可知:随着氧化过程的进行,溶液中Fe3+离子数目增多,溶液pH下降。影响黄铁矿氧化溶解的因素有很多,主要有溶液中溶解氧的浓度、溶液中铁离子浓度、黄铁矿颗粒表面积、颗粒表面不纯物质、温度等。表2所示为黄铁矿表面氧化溶出的铁离子浓度随pH的变化。由表2可知:黄铁矿在溶液中发生氧化,溶出了大量铁离子。随着pH降低,黄铁矿表面的铁离子溶出量升高。
表2 pH对铁离子溶出的影响
Table 2 Effect of pH on dissolution of Fe species

溶解的铁离子在溶液中不能稳定存在,在较高pH下会水解生成羟基铁和氢氧化铁,可以通过金属离子的溶液化学计算,绘出浓度为1×10-4 mol/L时铁离子在不同pH下各组分的浓度对数图,如图4 所示。由图4可知:在铁离子浓度为1×10-4 mol/L时,氢氧化铁在pH=2.9时开始产生沉淀。在氢氧化物沉淀之前,溶液中主要以荷正电的羟基金属离子存在。
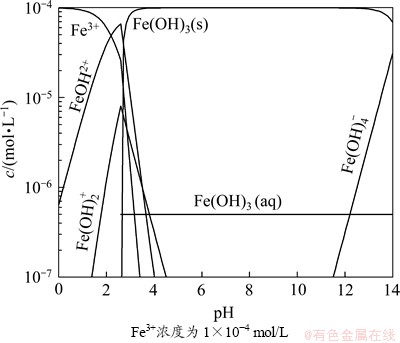
图4 Fe3+水解组分的浓度对数图
Fig. 4 Concentration logarithmic diagram of iron ion hydrolyzation components
由于溶液中氧气的存在,调浆过程中黄铁矿表面氧化,铁离子从黄铁矿表面溶解,溶解的铁离子容易水解形成羟基铁和氢氧化铁,吸附或沉淀在绿泥石表面,这将引起绿泥石电位发生变化,同时,表面氧化的发生也使黄铁矿的电位发生变化。图5所示为铁离子存在情况下,绿泥石电位随pH的变化,由图5可以看出:加入铁离子后,绿泥石的表面电位随着pH增加而增大,当增大到最大值后随着pH的增加而不断变小,在pH=9.6时表面电位变为0 mV。这是由于铁离子水解生成的氢氧化物或羟基物在绿泥石表面吸附的结果。许多研究均表明铁离子和铜离子在滑石等硅酸盐表面吸附会使硅酸盐矿物的表面电位发生相似的变化[19-21]。图5所示结果还显示了氧化的黄铁矿的表面电位随pH的变化。氧化使黄铁矿的等电点发生了移动,Fornasiero的研究发现表面完全氧化的黄铁矿的等电点出现在pH=7左右[15],因此,本研究所用的黄铁矿等电点出现在pH=4左右是由于表面部分氧化的结果。
图5 矿物Zeta电位与pH的关系
Fig. 5 Relationship between Zeta potential of minerals and pH
根据DLVO理论,矿物颗粒之间的聚集分散主要由矿物颗粒之间的静电作用能和范德华作用能决定。矿物表面电位的变化必然会影响矿物颗粒之间的静电作用能,从而影响颗粒之间的聚集分散状态。根据经典DLVO理论,异相矿物水基悬浮体中颗粒间相互作用总势能VT为
VT =VW+VE (4)
式中:VW为范德华作用能;VE为静电作用能。球形颗粒间范德华作用能VW的表达式为
 (5)
(5)
其中:
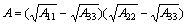 (6)
(6)
式中:A11为矿物1在真空中的Hamaker常数;A22为矿物2在真空中的Hamaker常数;A33为水在真空中的Hamaker常数;R1为矿物1球形粒子的半径;R2为矿物2球形粒子的半径;H为矿物1与矿物2颗粒间的距离。
半径分别为R1和R2的异相颗粒间的静电作用能VE的表达式为

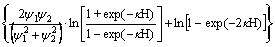 (7)
(7)
式中:ε0为真空中绝对介电常数,ε0=8.854×10-12 C2·J-1·m-1;εr为分散介质(水)的介电常数,εr=78.5 C2·J-1·m-1;y1与y2分别为矿物1与矿物2颗粒的表面电位,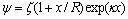 ;
; 为矿物固液界面Zeta电位;x为带电矿粒表面到滑移面的距离,取x=5×10-10 m;R为矿物颗粒半径,由表1可知绿泥石和黄铁矿的颗粒半径分别为4.91 μm和26.7 μm;
为矿物固液界面Zeta电位;x为带电矿粒表面到滑移面的距离,取x=5×10-10 m;R为矿物颗粒半径,由表1可知绿泥石和黄铁矿的颗粒半径分别为4.91 μm和26.7 μm; 为Debye长度,代表双电层厚度。
为Debye长度,代表双电层厚度。
根据式(4),可以得到pH=9时绿泥石与黄铁矿在水介质中颗粒间相互作用总势能与颗粒间距的关系,如图6所示。由图6可知:黄铁矿与绿泥石之间的相互作用能为正值,表明二者之间存在较强的相互排斥作用。黄铁矿表面氧化后,由于铁离子的氧化溶解以及重新沉淀吸附,绿泥石和黄铁矿的相互作用能变为负值,二者之间存在较强的相互吸引作用,容易发生异相凝聚。
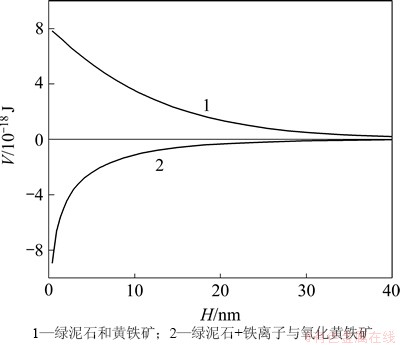
图6 绿泥石和黄铁矿在水介质中颗粒间相互作用总势能V与颗粒间距H的关系
Fig. 6 Relationship between interaction energy V of pyrite–chlorite particles and particles distance H
综合以上结果可以推测由于调浆时溶液中存在氧气,黄铁矿表面发生氧化,铁离子从黄铁矿表面溶解下来并发生水解,生成的羟基铁和氢氧化铁吸附在矿物表面,使其电位发生改变,黄铁矿和绿泥石由于电性相反而发生异相凝聚。
3 结论
1) 黄铁矿在实验所研究的pH范围内荷负电,未检测到等电点,绿泥石等电点约为4.5;当 pH大于4.5时,绿泥石和黄铁矿表面均荷负电。
2) 在溶液中有氧气存在的情况下进行调浆,黄铁矿表面氧化,铁从黄铁矿表面溶解下来,以三价铁形式进入溶液。
3) 溶液化学计算表明溶解的铁离子在溶液中水解形成羟基铁和氢氧化铁,这些组分会吸附或沉淀在矿物表面,导致黄铁矿和绿泥石的电位发生变化,使二者电性相反,发生异相凝聚。
参考文献:
[1] Feng B, Feng Q, Lu Y, et al. The effect of conditioning methods and chain length of xanthate on the flotation of a nickel ore[J]. Minerals Engineering, 2012, 39(12): 48-50.
[2] 马建青, 刘星. 甘肃金川铜镍矿石中MgO对浮选的影响[J]. 云南地质, 2005, 24(4): 402-406.
MA Jianqing, LIU Xing. Influence of MgO in ore of Jinchuan Cu-Ni deposit on floatation[J]. Yunnan Geology, 2005, 24(4): 402-406.
[3] Fornasiero D, Ralston J. Cu(II) and Ni(II) activation in the flotation of quartz, lizardite and chlorite[J]. International Journal of Mineral Processing, 2005, 76(1/2): 75-81.
[4] Pietrobon M C, Grano S R, Sobieraj S. Recovery mechanisms for pentlandite and MgO-bearing gangue minerals in nickel ores from Western Australia[J]. Minerals Engineering, 1997, 10(8): 775-786.
[5] 卢毅屏, 龙涛, 冯其明, 等. 微细粒蛇纹石的可浮性及其机理[J]. 中国有色金属学报, 2009, 19(8): 1493-1497.
LU Yiping, LONG Tao, FENG Qiming, et al. Flotation and its mechanism of fine serpentine[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(8): 1493-1497.
[6] Peng Y, Bradshaw D. Mechanisms for the improved flotation of ultrafine pentlandite and its separation from lizardite in saline water[J]. Minerals Engineering, 2012, 36(10): 284-290.
[7] Feng B, Feng Q, Lu Y. The effect of lizardite surface characteristics on pyrite flotation[J]. Applied Surface Science, 2012, 259(10): 153-158.
[8] LU Yiping, ZHANG Mingqiang, FENG Qiming, et al. Effect of sodium hexametaphosphate on separation of serpentine from pyrite[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(1): 208-213.
[9] Bremmell K,Fornasiero D, Ralston J. Pentlandite–lizardite interactions and implications for their separation by flotation[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2005, 252(2/3): 207-212.
[10] Feng B, Lu Y, Feng Q, et al. Talc–serpentine interactions and implications for talc depression[J]. Minerals Engineering, 2012, 32(5): 68-73.
[11] Forbes E, Davey K J, Smith L. Decoupling rehology and slime coatings effect on the natural flotability of chalcopyrite in a clay-rich flotation pulp[J]. Minerals Engineering, 2014, 56(2): 136-144.
[12] Bandini P, Prestidge C A, Ralston J. Colloidal iron oxide slime coatings and galena particle flotation[J]. Minerals Engineering, 2001, 14(5): 487-497.
[13] Edwards C R, Kipkie W B, Agar G E. The effect of slime coatings of the serpentine minerals; chrysotile and lizardite on pentlandite flotation[J]. International Journal of Mineral Processing, 1980, 7(1): 33-42.
[14] Alvarez-Silvaa M, Uribe-Salasb A, Mirnezami M, et al. The point of zero charge of phyllosilicate minerals using the Mular–Roberts titration technique[J]. Minerals Engineering, 2010, 23(5): 383-389.
[15] Peng Y, Zhao S. The effect of surface oxidation of copper sulfide minerals on clay slime coating in flotation[J]. Minerals Engineering, 2011, 24(15): 1687-1693.
[16] Chandra A P, Gerson A R. The mechanisms of pyrite oxidation and leaching: a fundamental perspective[J]. Surface Science Reports, 2010, 65(9): 293-315.
[17] Peng Y, Grano S. Effect of grinding media on the activation of pyrite flotation[J]. Minerals Engineering, 2010, 23(8): 600-605.
[18] Gu G, Sun X, Hu K, et al. Electrochemical oxidation behavior of pyrite bioleaching by Acidthiobacillus ferrooxidans[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(5): 1250-1254.
[19] 冯其明, 刘谷山, 喻正军, 等. 铁离子和亚铁离子对滑石浮选的影响及作用机理[J]. 中南大学学报(自然科学版), 2006, 37(3): 476-480.
FENG Qiming, LIU Gushan, YU Zhengjun, et al. Influence and mechanism of ferric and ferrous ions on flotation of talc[J]. Journal of Central South University (Science and Technology), 2006, 37(3): 476-480.
[20] 曹钊, 张亚辉, 孙传尧, 等. 铜镍硫化矿浮选中Cu(Ⅱ)和Ni(Ⅱ)离子对蛇纹石的活化机理[J]. 中国有色金属学报, 2014, 24(2): 506-510.
CAO Zhao, ZHANG Yahui, SUN Chuanyao, et al. Activation mechanism of serpentine by Cu(Ⅱ) and Ni(Ⅱ) ions in copper nickel sulfide ore flotation[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(2): 506-510.
[21] He M, Addai-Mensah J, Beattie D. Sericite–chalcocite mineral particle interactions and hetero-aggregation (sliming) mechanism in aqueous media[J]. Chemical Engineering Science, 2009, 64(13): 3083-3093.
(编辑 杨幼平)
收稿日期:2014-01-21;修回日期:2014-03-13
基金项目(Foundation item):国家自然科学基金资助项目(51404109);江西省教育厅科技项目(GJJ14425);江西省自然科学基金资助项目(20142BAB216021) (Project(51404109) supported by the National Natural Science Foundation of China; Project(GJJ14425) supported by the Education Department of Jiangxi Province; Project(20142BAB216021) supported by the Natural Science Foundation of Jiangxi Province)
通信作者:冯博,博士,讲师,从事矿物加工理论与工艺研究;E-mail: fengbo319@163.com