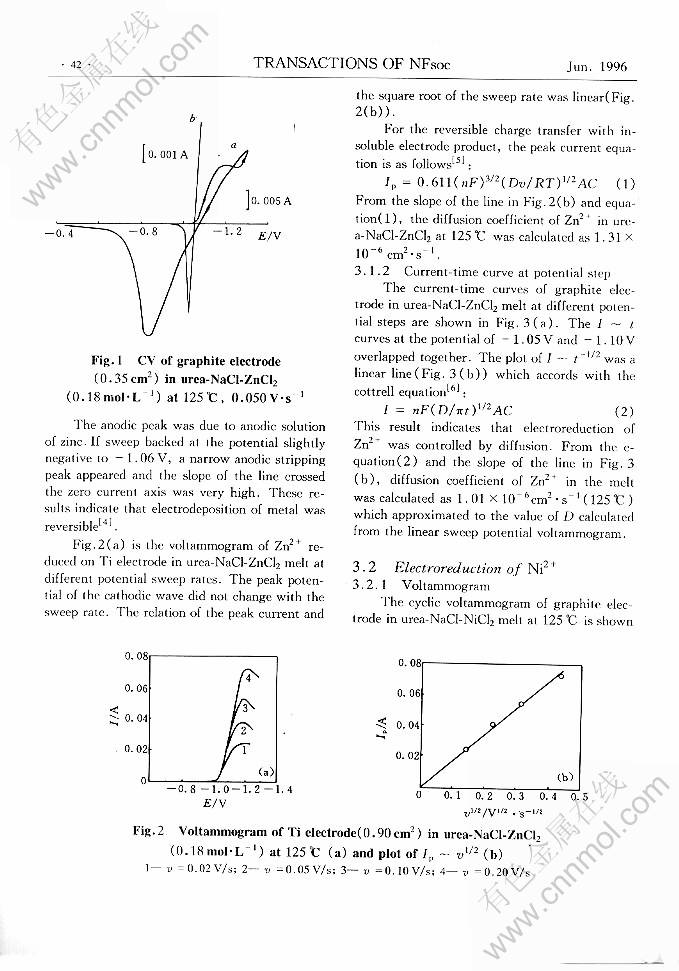
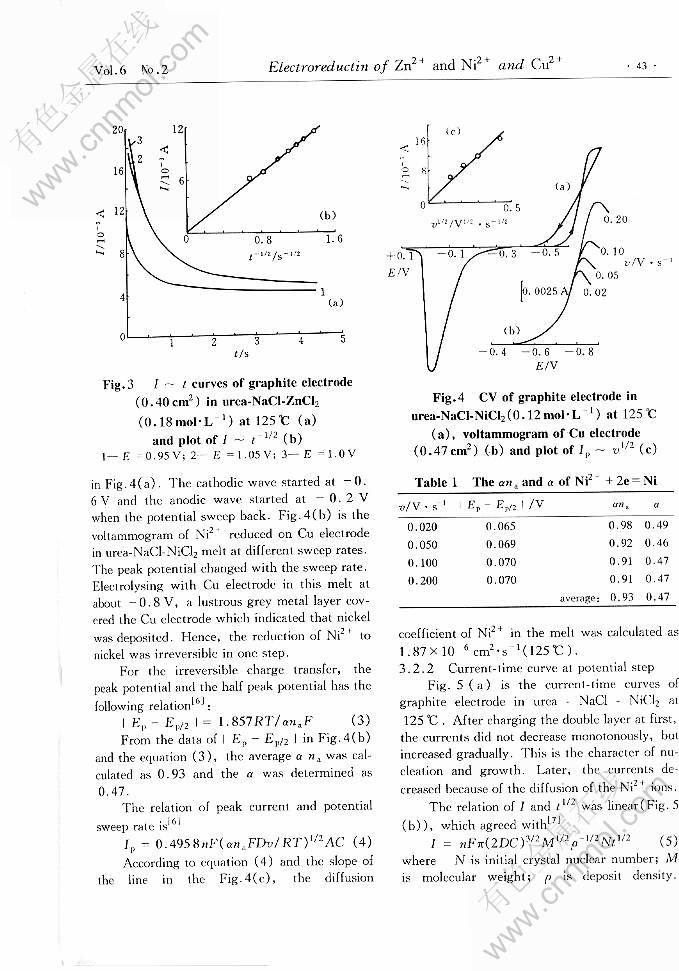
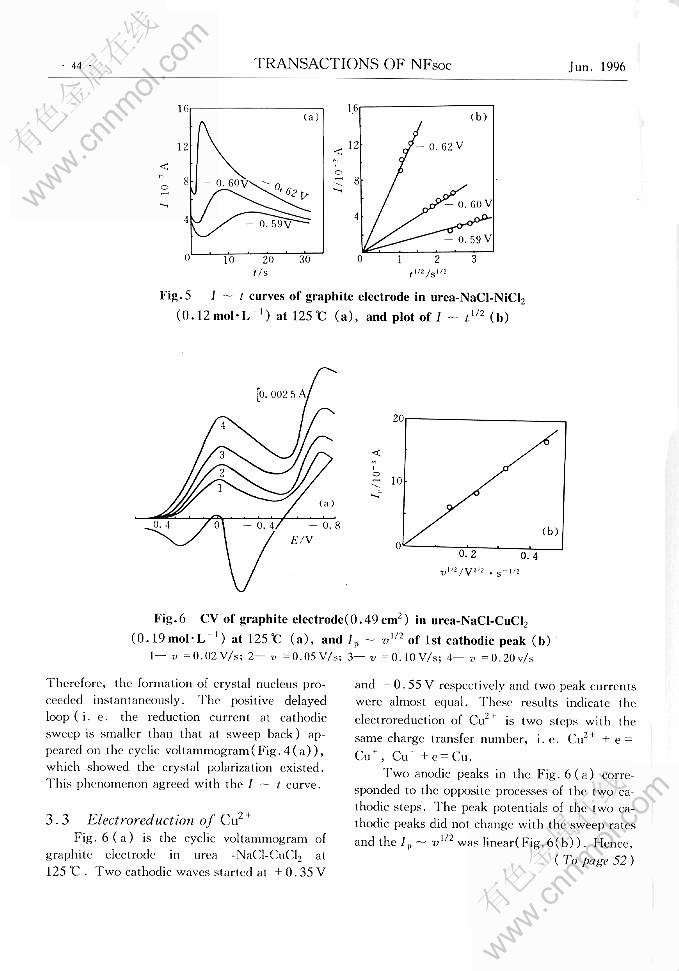
ELECTROREDUCTION OF Zn2+ AND Ni2+ AND Cu2+ IN UREA-CHLORIDES MELT
来源期刊:中国有色金属学报(英文版)1996年第2期
论文作者:Liu Peng Yang Qiqin Liu Guankun
文章页码:41 - 44
Key words:urea-chlorides melt; electroreduction of Zn2+, Ni2+and Cu2+; diffusion coefficient; transfer Coefficient
Abstract: The cyclic voltammetry, current-time curve at potential step were used to investigate the electroreduction of Zn2+, Ni2+ and Cu2+ in the urea-chlorides melt. Experimental results indicate that the electoreduction of Zn2+to zinc is reversible in one step, the electoreduction of Ni2+ to nickel is irreversible in one step and the electroreduction of Cu2+ to copper is reversible with two steps. The diffusion coefficients of Zn2+, Ni2+ and Cu2+ in urea-chlorides melt and the transfer coefficient of Ni2++2e→Ni were determined.