Trans. Nonferrous Met. Soc. China 24(2014) 2102-2106
Hydrogen emission at grain boundaries in tensile-deformed Al-9%Mg alloy by hydrogen microprint technique
Ryoto KOYAMA1, Goroh ITOH2
1. School of Science and Engineering, Ibaraki University, 4-12-1 Nakanarusawa, Hitachi, Ibaraki 316-8511, Japan;
2. College of Engineering, Ibaraki University, 4-12-1 Nakanarusawa, Hitachi, Ibaraki 316-8511, Japan
Received 17 October 2013; accepted 2 April 2014
Abstract: In recently years, environmental problems, such as global warming and exhaustion of fossil fuels, have grown into serious problems. In the automakers, the development of the fuel cell vehicles using hydrogen as clean energy has been paid attention to. Aluminum alloys have already been applied to a liner material of a high-pressure hydrogen tank for fuel cell vehicles. However, the behavior of hydrogen in aluminum alloys has not been clearly elucidated yet. Therefore, it is necessary to analyze the hydrogen behavior in aluminum alloys. Hydrogen microprint technique (HMPT) has been known as an effective measure to investigate the hydrogen behavior. In the present study, the emission behavior of internal hydrogen on a tensile-deformed Al-9%Mg alloy was investigated by HMPT at room temperature. As a result, the hydrogen was emitted at some grain boundaries.
Key words: aluminum-magnesium alloy; internal hydrogen; hydrogen microprint technique; grain boundary
1 Introduction
In recently years, environmental problems, such as global warming and exhaustion of fossil fuels, have grown into serious problems. Therefore, in the automakers, the development of the fuel cell vehicles using hydrogen as clean energy has been paid attention to. Currently, the 6061 aluminum alloys are used as a liner material of a high-pressure hydrogen tank for the fuel cell vehicles (FCVs). It is constantly required to use high-strength aluminum alloy in order to reduce the FCVs weight and cost. However, there are a lot of unknown parts for the hydrogen in aluminum alloy. Thus, it is necessary to analyze the behavior of hydrogen in aluminum alloy in order to guarantee safety.
In general, hydrogen in material can be divided into two kinds, internal hydrogen mixed into materials during manufacturing process, and environmental hydrogen intruding from environment during usage [1,2]. The method to visualize the microscopic location of hydrogen is hydrogen microprint technique (HMPT) [3,4]. The HMPT is the most convenient and safe technique [5].
In the previous study on Al-Mg alloy and pure aluminum of 99.99%, behavior of internal hydrogen emitted during plastic deformation was investigated by HMPT. It was reported that hydrogen was emitted from some of slip lines and grain boundaries [6-9]. However, mechanism of hydrogen emission at the grain boundary is unknown.
On the other hand, it has been reported that surface oxide film of the aluminum alloy blocks the emission of hydrogen [10].
In hydrogen emission from the grain boundary, we think that hydrogen was emitted by neighborhood of grain boundary, surface oxide film was destroyed by surface relief of specimen. In this study, the emission behavior of internal hydrogen was investigated when an Al-Mg alloy was tensile-deformed at room temperature. The relationship of the hydrogen emission from grain boundaries to the surface relief caused by the deformation was studied.
2 Principle of HMPT
In HMPT, a hydrogen atom emitted on the specimen surface is visualized as a silver particle using photographic emulsion layer containing silver bromide. The principle of HMPT is schematically shown in Fig. 1. Following the oxidation-reduction reaction indicated in the following formula, the metallic silver atom will be produced by a strong reduction power of the hydrogen atom at the site where the hydrogen atom is emitted.
Ag++H→Ag+H+
It was reported that the amount of hydrogen emission in Al-Mg alloy is very small [8]. Therefore, the specimen was soaked in developer. By this process, the whole silver bromide particle will become metallic silver. The silver bromide particles that have not reacted with hydrogen will dissolve out into the fixer, and finally only the metallic silver particles will remain on the specimen surface in gelatin film of the emulsion. Hence, it is possible to visualize the emission site of hydrogen by observing the distribution of the remaining silver particles together with metallographic microstructure.
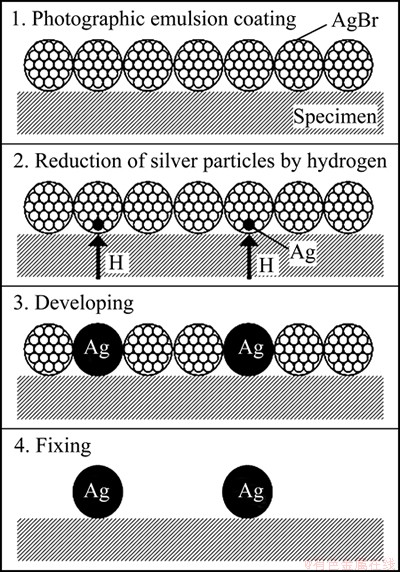
Fig. 1 Principle of hydrogen microprint technique
3 Experimental
An Al-9%Mg alloy was prepared from a pure aluminum of 99.99% and pure magnesium of 99.9%. It melted in air using MgCl2 as a flux and cast in an iron mold. The resulting ingot with 26.5 mm in thickness was homogenized at 430 °C for 18 h. Table 1 shows the chemical composition of the ingot. The ingot was then warm-rolled at 200 °C to a 1.1 mm-thick sheet. The sheet was annealed at 450 °C for 10 min, air-cooled and then cold-rolled to a 1.0 mm-thick sheet. Tensile test pieces were cut from the sheet in L direction. Figure 2 shows the detail of the test piece. After that, the specimen was heat-treated. The test pieces were either furnace-cooled after annealing at 400 °C for 1 h, or water-cooled after solution heat treatment at 500 °C for 10 min. Then, they were polished by wet abrasive paper up to #1500, and finally electro-polished at the gage portion after etching. The electrolysis solution was 14% distillated water, 80% ethanol and 6% perchloric acid.
Table 1 Chemical composition of ingot (mass fraction, %)

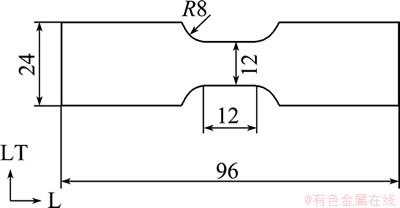
Fig. 2 Schematic diagram and dimension of tensile test piece with 1.0 mm in thickness (unit: mm)
Next, the gage portion of test piece was coated with collodion film to improve the adhesion of emulsion to the specimen. Then, it was covered with photographic emulsion diluted four times on the surface by the wire-loop method in a dark room, naturally dried, and finally clamped at the experimental device shown in Fig. 3, where the emulsion-coated surface was shielded from light.
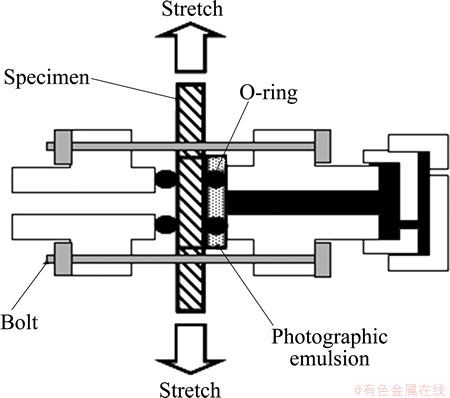
Fig. 3 Experimental device for stretching with photographic emulsion film shielded from light
The test pieces were stretched at room temperature by a screw-driven testing machine. In this experiment, three kinds of specimens with plastic strain of 9%, 12% and 21% were prepared. Then, three specimens were soaked in developer, rinsed with tap water, fixed in formalin and sodium thiosulfate aqueous solution, rinsed again with tap water and naturally dried.
Finally, each gage portion of the specimens covered with photographic emulsion was observed by a scanning electron microscope (SEM) equipped with an energy dispersive X-ray spectroscopy (EDXS) device. Furthermore, surface relief of the specimens was measured by a laser microscope.
4 Results and discussion
After electro-polishing the gage portion, we observed the metallographic structure by an optical microscope. Figure 4 shows the optical micrographs of the two specimens, i.e., annealed specimen: furnace- cooled after annealing at 400 °C for 1 h; quenched specimen: water-cooled after solution heat treatment at 500 °C for 10 min. It can be observed a large number of precipitates (Al3Mg2) at grain boundaries in the annealed specimen. The precipitates can be hydrogen trapping sites. Therefore, it was impossible to conclude whether hydrogen in the matrix was emitted at the grain boundaries because the hydrogen transport with plastic deformation or trapped hydrogen was simply emitted by the deformation. On the other hand, no precipitate was observed at the grain boundaries of quenched specimen. Therefore, we observed the emission of internal hydrogen in the specimen quenched and subsequently stretched at room temperature.
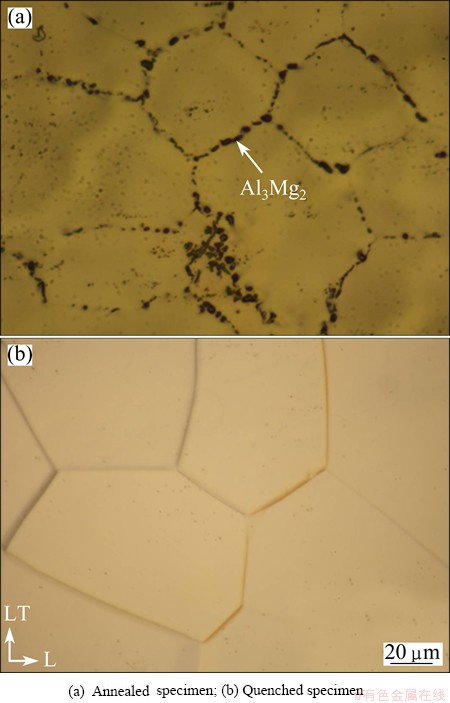
Fig. 4 Optical micrographs of two specimens
Figure 5 shows the HMPT/SEM images. A number of white particles were observed at some grain boundaries of the three specimens. A result of EDXS on one of the white particles is shown in Fig. 6. The peaks of Al and Ag are confirmed, indicating that these white particles are silver particles. Therefore, in all the specimens, it is concluded that the hydrogen is emitted at some grain boundaries.
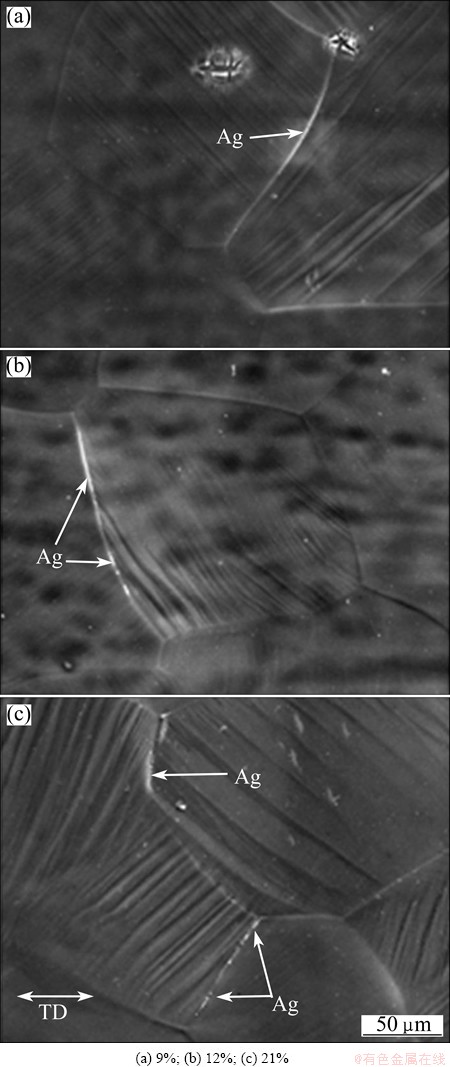
Fig. 5 HMPT/SEM images of three specimens stretched at room temperature with different plastic strains
Then, the number of grain boundaries with hydrogen emission was counted based on HMPT/SEM images of three specimens. As a result, the more the tensile deformation amount, the greater the number of grain boundaries with hydrogen emission.
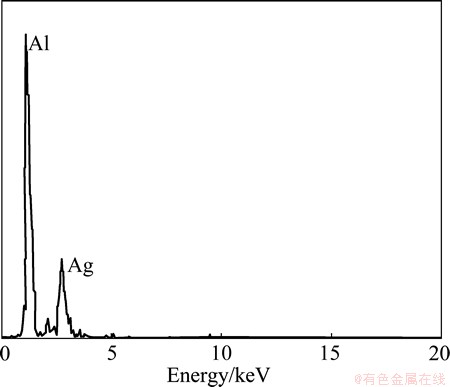
Fig. 6 EDXS spectrum taken from white particle shown in Fig. 5(a)
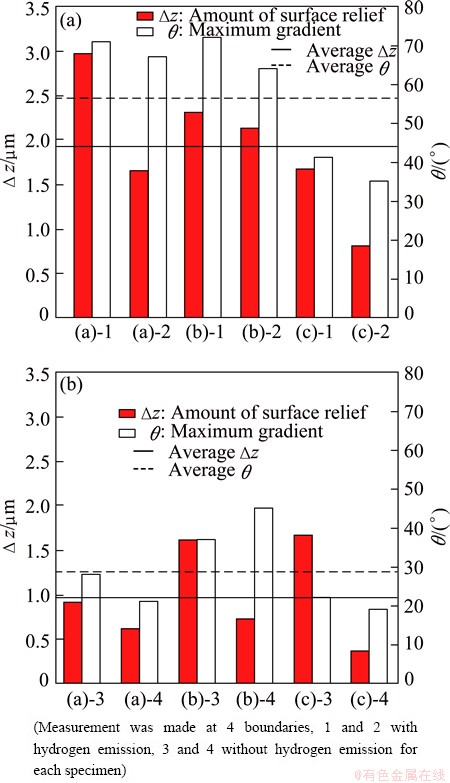
Fig. 7 Difference in height between two neighboring grains, Δz, and maximum gradient angles, θ1, in surface profile for grain boundaries with (a) or without (b) hydrogen emission
Figure 7 shows the difference in the height between the two neighboring grains and the maximum gradient across the grain boundary (with or without hydrogen emission). In Fig. 7, it is found that the amount of surface relief and the maximum gradient of the grain boundary with hydrogen emission are larger than those without hydrogen emission. It has been reported that surface oxide film thickness of aluminum with 99.99% purity after electro-polishing is 2-5 nm. It is thought that the thickness of the surface oxide film on the Al-Mg alloy is the same as this. Therefore, we think that surface oxide film at grain boundaries was cracked by the formation of great surface relief and gradient, and hence the hydrogen was emitted from grain boundaries.
In spite of the great surface relief and gradient, we have recognized some grain boundaries without hydrogen emission. We thought that the reason for this hydrogen emission is associated with local crystal orientation around grain boundaries and deformation mode in the grains. Figure 8 shows the angle of grain boundaries with hydrogen emission against tensile direction (θ1) and against slip line (θ2). It is to be noted that most grain boundaries with hydrogen emission are from 46° to 75° to tensile direction, and are nearly parallel to slip lines.
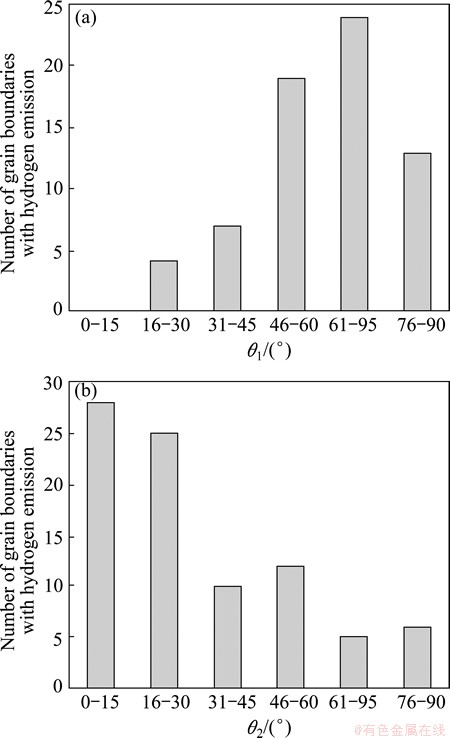
Fig. 8 Angle of grain boundaries with hydrogen emission against tensile direction (θ1) and against slip line (θ2)
5 Summary
Relationship between the hydrogen emission from grain boundaries and the surface relief caused by the deformation was investigated. It was confirmed that the amount of surface relief and the maximum gradient across the grain boundary with hydrogen emission are larger than those without hydrogen emission. Thus, we presumed that surface oxide film at the grain boundaries was cracked by the formation of large surface relief and gradient, allowing hydrogen to be emitted from the grain boundaries.
References
[1] ITOH G, KANNO M. Hydrogen in aluminum [J]. Metals and Technology (Kinzoku), 1996, 66(7): 599-610.
[2] NAGAO A, KURAMOTO S, KANNO M. Detection and visualization of hydrogen in aluminum [J]. Light Metals, 1999, 49: 89.
[3] KOYAMA K, ITOH G, KANNO M. Visualizing technique of impurity hydrogen evolved from aluminum during deformation [J]. Journal of Japan Institute of Metals, 1997, 61(4): 366-367.
[4] KOYAMA K, ITOH G, KANNO M. Observation of impurity hydrogen evolved from aluminum and titanium alloys during deformation by means of hydrogen microprint technique [J]. Journal of Japan Institute of Metals, 1998, 62(9): 791-795.
[5] KURAMOTO S, ICHITANI K, NAGAO A, KANNO M. Effect of gelatin hardening on hydrogen visualization in steels by hydrogen microprint technique [J]. Tetsu-to-Hagan, 2000, 86(1): 17-23.
[6] IHARA T, ITOH G. Behavior of hydrogen in Al-Mg alloys investigated by means of hydrogen microprint technique [J]. Journal of Japan Institute of Light Metals, 2003, 53: 575-581.
[7] HORIKAWA K, YOSHIDA K. Visualization of hydrogen distribution in tensile-deformed Al-5%Mg alloy investigated by means of hydrogen microprint technique with EBSD analysis [J]. Journal of Japan Institute of Metals, 2004, 68(12): 1043-1046.
[8] IZUMI T, ITOH G. Behavior of environmental hydrogen in high-magnesium Al-Mg alloys analyzed by hydrogen microprint technique [J]. Journal of Japan Institute of Light Metals, 2006, 56: 478-482.
[9] KOYAMA K, ITOH G, KANNO M. Observation of impurity hydrogen evolved from aluminum during deformation by means of silver decoration technique [J]. Journal of Japan Institute of Metals, 1998, 62(8): 742-747.
[10] UMEDA H, ITOH G, KATO Y. Effect of heat treatment condition on the hydrogen content in Al-4%Mg alloys [J]. Journal of Japan Institute of Light Metals, 2006, 56(4): 203-209.
基于氢微缩技术拉伸变形Al-9%Mg合金的晶界氢析出
Ryoto KOYAMA1, Goroh ITOH2
1. School of Science and Engineering, Ibaraki University, 4-12-1 Nakanarusawa, Hitachi-City, Ibaraki 316-8511, Japan;
2. College of Engineering, Ibaraki University, 4-12-1 Nakanarusawa, Hitachi-City, Ibaraki 316-8511, Japan
摘 要:近年来,诸如全球变暖、化石燃料枯竭等环境问题日趋严重。发展使用氢作为清洁能源的燃料电池汽车,受到了汽车制造商的广泛重视。铝合金已经应用到燃料汽车的高压氢气槽的衬垫材料中。然而,氢在铝合金中的反应仍然没有得到清楚的阐明。因此,有必要分析氢在铝合金中的反应。氢缩影技术(HMPT)一直被视为一种探讨铝合金中氢行为的有效措施。目前,在室温下采用HMPT技术研究了拉伸变形Al-9%Mg合金内部氢析出的过程,发现氢在一些晶界处析出。
关键词:铝镁合金;内部氢析出;氢缩影技术;晶界
(Edited by Xiang-qun LI)
Corresponding author: Goroh ITOH; E-mail: gitoh@mx.ibarki.ae.jp
DOI: 10.1016/S1003-6326(14)63318-5