J. Cent. South Univ. (2012) 19: 1802-1807
DOI: 10.1007/s11771-012-1211-2
Crystallization of calcium carbonate in hydrogels in presence of meso-tetrakis (4-hydroxylphenyl) porphyrin
ZHANG Feng-ju(张凤菊)1, YANG Xin-guo(杨新国)1, 2, ZHUANG Yu(庄钰)1,
GUO Kun-kun(郭坤琨)1, WANG Ming-jun(王明军)1, WEI Wen-feng(魏文峰)1
1. College of Materials Science and Engineering, Hunan University, Changsha 410082, China;
2. State Key Laboratory of Silicon Materials (Zhejiang University), Hangzhou 310027, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: Organic matrices play an important role in biomineralization process. In order to explore the effect of both meso-tetrakis (4-hydroxylphenyl) porphyrin (THPP) and hydrogels on calcium carbonate mineralization, and consequently synthesize functional materials based on porphyrin and calcium carbonate with tunable shapes and optical properties, a new kind of biomimetic mineralization system which combined THPP with three biopolymer hydrogels (gelatin, agarose and calcium alginate gels) was designed and investigated. A carbonate diffusion method based on the generation of CO2 by slow decomposition of ammonium hydrogen carbonate was adopted for calcium carbonate crystallization. The results show that both gelatin and alginate hydrogels exhibit the ability of stabilizing vaterite, while agarose only induces the formation of calcite. With participation of THPP in the mineralization environments, calcite is favored in all these hydrogels, while the crystal morphologies are greatly different from each other. These results indicate the perspective of THPP in regulating calcium carbonate crystallization and also provide a new strategy for fabricating advanced functional materials with controlled morphology and tunable optical properties based on calcium carbonate and THPP.
Key words: tetra-p-hydroxyphenyl porphyrin; hydrogel; calcium carbonate; biomimetic mineralization
1 Introduction
Biominerals usually possess unique shapes and properties. It is generally assumed that organic matrices play a key role in the mineralization process. During the past two decades, great efforts have been concentrated on biomimetic mineralization. Calcium carbonate is one of the most abundant biominerals produced by organisms and also has industrial applications due to its wide use as filler in paints, plastics, rubber, or paper. Calcium carbonate occurs in three main crystalline polymorphs: vaterite, aragonite and calcite in the order of decreasing solubility and increasing stability. Organic additives or templates such as double-hydrophilic block copolymers [1-2], dendrimers [3], common polymer [4], synthetic peptides or amino acids [5-6], and biomacromolecules [7] have been used to induce the controlled growth and crystallization of CaCO3 mineral, as well as for control of the polymorphs. However, the research related to introducing the biomineralization notion into preparation of functional materials is very limited. Recently, SINDHU et al [8] reported that luminescent polymers poly (p-phenylene) can be incorporated into calcium carbonate crystals and the obtained composites showed improved photostability.
In the present work, meso-tetrakis (4-hydroxylphenyl) porphyrin (THPP), a porphyrin compound with interesting optical and electronic properties, was used to induce calcium carbonate crystallization in three biopolymer hydrogels. Investigations of CaCO3 mineralization in gelatin, alginate and agarose hydrogels have been carried out previously [9-11]. However, systematic comparison between various hydrogels about their influence on calcium carbonate crystallization, and the mineralization mechanisms are still far from being fully understood. On the other hand, porphyrins have attracted much attention due to their outstanding photophysical, photochemical and electronic properties, which make them promising alternatives in areas of nonlinear optical materials, optical deposit elements, photodynamic therapy of cancer, etc [12-13]. Various porphyrin derivatives with special peripheral substitutes and functional properties can be prepared via organic synthesizing or by chemical modification. With easily modified hydroxyl groups on its molecular structure (Fig. 1(a)), THPP is such an important dye intermediate that many porphyrin compounds may be synthesized from it.
The aim of this work is to synthesize functional materials based on porphyrin and calcium carbonate with tunable shapes and optical properties, and to explore the effect of both THPP and hydrogels on the calcium carbonate mineralization.
2 Experimental
2.1 Materials
The hydrogels used in this work were prepared from gelatin (Sinopharm Chemical Reagents Co. Ltd., Shanghai, China), agarose (Biochemical Reagents, Shanghai Yuanju Biotechnology Ltd., China) and sodium alginate (Wenzhou Agents Factory, Zhejiang Province, China). CaCl2 and (NH4)2CO3 were analytical reagents and were purchased from Damao Chemical Reagent Factory and Laiyang Fine Chemical Plant (China), respectively. THPP was prepared by the procedure according to Ref. [14]. Deionized water was used throughout sample preparations. Chemical structures of p-THPP, gelatin, agarose and alginate are shown in Fig. 1.
The decomposition reaction of ammonium hydrogen carbonate is
NH4HCO3=NH3+CO2+H2O (1)
2.2 Preparation of control sample
A carbonate diffusion method based on the generation of CO2 by slow decomposition of ammonium hydrogen carbonate was adopted in this work for calcium carbonate crystallization. 1% (w/v) CaCl2 aqueous solution was heated to 40 ℃ and gelatin powder was added under stirring to get gelatin content of 3% (w/v). The resulting hot solution was poured into petri dishes and cooled to room temperature naturally to form hydrogel. After that, hydrogels with inclusion of CaCl2 were placed in a closed desiccator containing crushed ammonium carbonate (analytical reagent, Laiyang Fine Chemical Plant, China) at the bottom. All the crystallization procedures in this work were carried out at room temperature by slow diffusion of CO2 into the hydrogels containing CaCl2. Samples were taken out of the desiccator after certain time and were washed by deionized water at 80 ℃. After centrifugation, the white precipitates were collected, dried in a vacuum oven at 40 ℃ for 48 h and then sealed in a desiccator before being analyzed. Agarose gels were prepared from agarose solutions with content of 1% (w/v). The process of CaCO3 crystallization in agarose gels was the same as that in gelatin hydrogels except that the dissolving temperature was 80 ℃.
As for calcium carbonate crystallization in alginate hydrogels, sodium alginate was firstly dissolved in deionized water to form 3% (w/v) solution, then, calcium alginate hydrogel membranes were prepared as described by ZHANG et al [15] without the emulsification related steps. The rest mineralization processes were the same as those described above. The mineralization time in this work was 3 d if there was no special annotation.
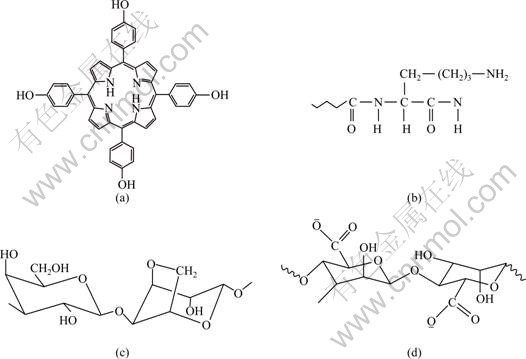
Fig. 1 Chemical structures of p-THPP (a), gelatin (b), agarose (c) and alginate (d)
2.3 Calcium carbonate mineralization in presence of THPP
THPP was mixed with gelatin solution, agarose solution and sodium alginate solution, respectively, by stirring to get suspensions with THPP content of 0.1% (w/v). The left processes were similar with that of the control sample preparation. Precipitates grown in the presence of THPP looked yellow green.
2.4 Crystal characterization
The prepared CaCO3 powders without gold coating were used to take electron micrographs through environmental scanning electron microscopy (ESEM) (FEI QUANTA 200). The X-ray diffraction (XRD) patterns were taken from powdered samples using a Siemens D-500 (Germany) generator. XRD scans were recorded in θ-θ geometry using Cu Kα radiation (λ= 1.54 ?). Correlation of crystal habit with polymorph type was determined by comparing SEM and XRD data: Rhombohedra and spherulitic morphologies were identified as calcite and vaterite, respectively.
3 Results
3.1 Calcium carbonate crystallization in gelatin hydrogel with and without THPP
Figure 2(a) shows the micrograph of CaCO3 grown in gelatin hydrogel. The crystals showed rhombohedral and spherical morphology. The particle size was in the range of 5-15 μm. These results were different from those reported previously [9], in which a double diffusion method was adopted and the products were barrel-shaped particles with size of more than 300 μm. The differences implied that experimental apparatus and calcium carbonate generating process (gelatin type, pH, temperature, concentration of the gel, etc) were all responsible for the morphology and polymorphs of the resulting minerals. The XRD patterns (Fig. 3(a)) demonstrated that the samples were mixtures of vaterite and calcite. The vaterite fraction was about 50% evaluated according to Eq. (2):
? (2)
(2)
where Ic104/Iv110 represents the ratio of the intensities of the reflection peaks at (104) for calcite to (110) for vaterite in a mixture of the two substances, and Xc/Xv is the molar fraction ratio of the two substances [16].
Generally, calcite rhombohedra were formed in control sample containing no organic matrix. The presence of vaterite crystals indicated that gelatin hydrogel stabilized the less thermodynamically stable CaCO3 polymorph.
When THPP was introduced into gelatin hydrogel, to our surprise, the particles had several smooth and ordered planes (Fig. 2(b)), and the particle size was 8-25 μm. XRD pattern (Fig. 3(b)) exhibited peaks at 2θ=27.1° corresponding to the (132) plane of vaterite, as well as peaks at 2θ=23.1, 2θ=29.5° assigned to (012), (104) planes of calcite. While the diffraction peak of vaterite (024) (2θ=32.7°) disappeared as compared with that in the absence of THPP (Fig. 3(a)). Another notable phenomenon is that the particles obtained in the presence of THPP were enlarged as compared with those obtained in the absence of THPP. Considering the fact that multi-hydroxyl groups of THPP can interact with both crystal faces and functional groups on gelatin molecules, it is reasonable that THPP or the complex of THPP and were gelatin molecules adsorbed onto crystal surfaces, altering the stability of that face and hence affecting the growth rate of particles. And the larger crystal particles indicated that a chemical microenvironment favoring crystal growth was provided with the addition of THPP into gelatin hydrogels.

Fig. 2 Scanning electron micrographs of calcium carbonate crystals grown in gelatin hydrogel: (a) In the absence of THPP; (b) In the presence of THPP
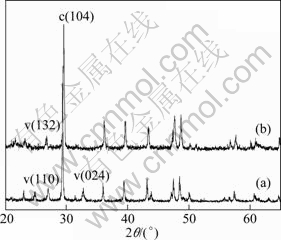
Fig. 3 XRD patterns of calcium carbonate obtained in gelatin hydrogels: (a) In the absence of THPP; (b) In the presence of THPP
3.2 Calcium carbonate crystallization in agarose hydrogel with and without THPP
Agarose forms a gel when a homogenous solution is cooled to below the ordering temperature (~35 ℃) and an infinite three-dimensional network of agarose fibers develops; the melting of agarose gel occurs at comparatively higher temperature (~85 ℃). Previous study [10] predicted that the main role that agarose gels playing on CaCO3 crystallization was the control on the diffusion process of reactant ions rather than the hindering of crystal growth. This was confirmed by considering the pore size for 1% agarose gels of around 140 nm [17], considerably smaller than the initial rhombohedral calcite crystals. The present study results are in agreement with the conclusion mentioned above. The samples formed in agarose with or without THPP were all composed of cubical crystals (Fig. 4). There was dramatic change in particle size as compared with the products obtained in agarose hydrogel with and without THPP. XRD patterns (Fig. 5) showed that the growth of calcite crystal plane (012) was suppressed in agarose gels and was favored with the presence of THPP. It seemed that THPP changed the chemical environment of mineralization in agarose hydrogel and so changed the growth habit of crystal planes.

Fig. 4 Scanning electron micrographs of calcium carbonate crystals grown in agarose hydrogels: (a) In the absence of THPP; (b) In the presence of THPP

Fig. 5 XRD patterns of calcium carbonate crystals grown in agarose hydrogels: (a) In the absence of THPP; (b) In the presence of THPP
3.3 Calcium carbonate crystallization in alginate hydrogel with and without THPP
Alginate is a natural block copolymer extracted from seaweeds, composed of linear unbranched polymers containing β-(1-4)-linked D-mannuronic acid and α-(1-4)-linked L-guluronic acid (Fig. 1(d)). The influence of sodium alginate on calcium carbonate crystallization has been reported previously [18]. The obtained CaCO3 was rosette-like aggregate. This morphology formation was suggested to be due to the strong interactions between carboxyl groups on alginate molecules and calcium ions. Recently, calcium alginate hydrogel as the template of CaCO3 mineralization was reported [11] and the samples were composed of single-crystal particles of calcite and calcite superstructures.
In this work, calcium carbonate mineralization in alginate hydrogel was induced by carbonate diffusion method. Carbon dioxide and ammonia were produced by decomposition of ammonium carbonate. During the mineralization process, CO2 diffused into calcium alginate membrane and interacted with Ca2+ both on the hydrogel network points and inside the hydrogel networks to precipitate calcium carbonate. At the same time, NH3 dissolved in the water was encapsulated in hydrogel membrane and replaced Ca2+ conjugated by —COO— on alginate macromolecules. These interactions led to the collapse of calcium alginate hydrogel membrane. Thus, CaCO3 crystallization actually took place in alginate hydrogel at the beginning and then in a sol-like system, just as observed during the mineralization process. Anyway, the present work still showed evident influence of alginate on CaCO3 morphology and polymorph, and the results were different from the previous report [11]. CaCO3 precipitation in alginate system in the absence of THPP gave birth to microspheres with rough texture (Fig. 6(a)). The particle size was 5-10 μm. XRD pattern (Fig. 7(a)) showed both calcite and vaterite diffraction peaks. CaCO3 precipitation in alginate system in the presence of THPP resulted in spherical superstructures decorated with orderly aggregated small rhombohedrons (Fig. 6(b)). The spherulites were 10-20 μm in diameter, much larger than those in the absence of THPP, and the size of rhombohedra subcrystals was 1-2 μm. XRD pattern (Fig. 7(b)) indicated that these spherical aggregates were dominantly calcite. This spherical superstructure was similar with that reported by BUTLER et al [19], but the subcrystals arrangements were different. It was suggested that the spherical crystal morphology and the special arrangement of subcrystals were related to the special interactions between THPP and alginate and their influences on crystal growth via an adsorption mechanism.
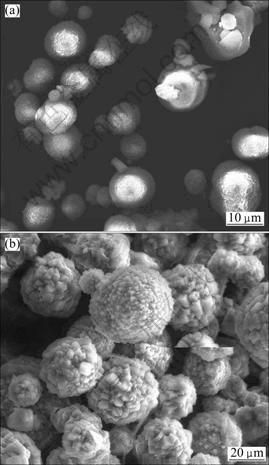
Fig. 6 Scanning electron micrographs of calcium carbonate crystals grown in alginate hydrogels: (a) In the absence of THPP; (b) In the presence of THPP

Fig. 7 XRD patterns of calcium carbonate crystals grown in alginate hydrogels: (a) In the absence of THPP; (b) In the presence of THPP
4 Discussion
There are ionizable functional groups on both gelatin and alginate molecules, while agarose only contains hydroxyl groups. The current results demonstrate that gelatin and alginate are more effective in stabilizing the less stable vaterite than agarose. Without the addition of THPP, both vaterite and calcite formed in gelatin and alginate hydrogels are in their typical morphologies, that is, spheres for vaterite and rhombohedra for calcite.
Previous investigation on CaCO3 crystallization in gelatin hydrogel and poly-acrylamide hydrogel revealed that the addition of poly-aspartate to the pore solution in either gelatin or poly-acrylamide hydrogel overcompensated the physical properties of the organic matrix, leading to the morphologies independent of hydrogel used [9].
In the present work, with inclusion of THPP in these hydrogels, the crystal morphologies are much complicated and are different from each other. In light of these results, it is proposed that THPP and each of the three hydrogels conjugate together and influence crystal growth corporately. Because of the specific characteristics of biopolymer molecular structures, their interactions with THPP vary from each other, thus leading to the differences in inducing calcium carbonate growth and aggregation. With inclusion of THPP in hydrogels, the size of the resulted particles increases markedly. It seems that CaCO3 is easier to deposit in hydrogels with inclusion of THPP, hence, the crystal pieces tend to grow larger and larger. Another explanation is that the absorbed small THPP molecules on the growing crystal faces serve as bridges, by which crystal pieces are united to form large particles. As the precipitation environments are different in these three kinds of hydrogels, the crystal pieces arrange in different manners. Therefore, the resulted morphologies are distinct from each other.
As for the effect of THPP on the selection of crystal polymorphs, the present results show that calcite is favored in all these three hydrogels with inclusion of THPP. The ability of stabilizing less stable vaterite for alginate and gelatin is shielded by THPP. The dominant factor in the crystal polymorph selectivity of calcium carbonate is still uncertain. Previous reports ascribed the driving force to surface charge densities [20], or to the CO2 escape rate [21]. It is speculated that both the organic templates and the biomineralization apparatus exert influences on polymorph selectivity. Calcite polymorph selection in this work is proposed to be due to the interactions between hydroxyl groups on THPP and the carboxyl or amino groups on gelatin or alginate molecules. These interactions weaken the influence of ionic groups on crystal growth and highlight the role of hydroxyl groups on crystal growth and polymorph selection. Further investigation on the mineralization mechanism is in progress.
5 Conclusions
1) Both gelatin and alginate hydrogels exhibit the ability of stabilizing vaterite, while agarose only induces the formation of calcite. Participation of THPP in the mineralization environments favors the formation of calcite, while the crystal morphologies are greatly different from each other.
2) The interactions between THPP and hydrogels and their cooperating influences are responsible for the resulted crystal polymorph selection and complex structures.
References
[1] C?LFEN H, ANTONIETTI M. Crystal design of calcium carbonate microparticles using double-hydrophilic block copolymers [J]. Langmuir, 1998, 14: 582-589.
[2] YU Shu-hong, C?LFEN H, TAUER K, ANTOIETTI M. Tectonic arrangement of BaCO3 nanocrystals into helices induced by a racemic block copolymer [J]. Nat Mater, 2005, 4: 51-55.
[3] NAKA K. Effect of dendrimers on the crystallization of calcium carbonate in aqueous solution [J]. Top Curr Chem, 2003, 228: 83-112.
[4] HAN J T, XU X R, KIM D H, CHO K. Biomimetic fabrication of vaterite film from amorphous calcium carbonate on polymer melt: Effect of polymer chain mobility and functionality [J]. Chem Mater, 2005, 17: 136-141.
Effect of polymer chain mobility and functionality [J]. Chem Mater, 2005, 17: 136-141.
[5] MANOLIA F, KANAKISA J, MALKAJB P, DALAS E. The effect of aminoacids on the crystal growth of calcium carbonate [J]. J Cryst Growth, 2002, 236: 363-370.
[6] QIAO Li, FENG Qing-ling, LI Zhou, LU Shan-shan. Alternate deposition of oriented calcite and amino acid layer on calcite substrates [J]. J Phys ChemB, 2008, 112: 13635-13640.
[7] FALINI G, FERMANI S, GAZZANO M, RIPAMONTI A. Oriented crystallization of vaterite inside collagenous matrices [J]. Chem Eur J, 1998, 4: 1048-1952.
[8] SINDHU S, JEGADESAN S, HAIRONG L, AJIKUMAR P K, VETRICHELVAN M, VALIYAVEETTIL S. Synthesis and patterning of luminescent CaCO3–poly (p-phenylene) hybrid materials and thin films [J]. Adv Funct Mater, 2007, 17: 1698-1704.
[9] GRASSMANN O, M?ller G, L?BMANN P. Organic-inorganic hybrid structure of calcite crystalline assemblies grown in a gelatin hydrogel matrix: relevance to biomineralization [J]. Chem Mater, 2002, 14: 4530-4535.
[10] YANG Dong, QI Li-min, MA Ji-ming. Well-defined star-shaped calcite crystals formed in agarose gels [J]. Chem Commun, 2003, 10: 1180-1181.
[11] LI Xin-ping, SHEN Qiang, SU Yun-lan, TIAN Fang, ZHAO Ying, WANG Du-jin. Structure-function relationship of calcium alginate hydrogels: A novel crystal-forming engineering [J]. Crys Growth Des, 2009, 9: 3470-3476.
[12] WEBSTER S, ODOM SA, PADILHA LA, PRZHONSKA OV, PECELI D, HU H, NOOTZ G, KACHKOVSKI AD, MATICHAK J, BARLOW S, ANDERSON HL, MARDER SR, HAGAN DJ, VAN SEW. Linear and nonlinear spectroscopy of a porphyrin-squaraine- porphyrin conjugated system [J]. J Phys Chem B, 2009, 113: 14854-14867.
[13] YAMAMURA T, SUZUKI S, TAGUCHI T, ONODA A, KAMACHI T, OKURA I. Porphyrin arrays responsive to additives: Fluorescence tuning [J]. J Am Chem Soc, 2009, 131: 11719-11726.
[14] GUO Xi-liang, AN Wen-ting, SHUANG Shao-min, CHENG Fang-qin, DONG Chuan. Study on spectroscopic characterization of meso-tetrakis (4-hydroxyphenyl) porphyrin (THPP) in β-cyclodextrin and its derivatives [J]. J Photoch Photobio A: Chem, 2005, 173: 258-263.
[15] ZHANG Feng-ju, CHENG Guo-xiang, GAO Zhi, LI Cui-ping. Preparation of porous calcium alginate membranes/microspheres via an emulsion templating method [J]. Macromol Mater Eng, 2006, 291: 485-492.
[16] KONTOYANNIS C G, VAGENAS N V. Calcium carbonate phase analysis using XRD and FT-Raman spectroscopy [J]. Analyst, 2000, 125: 251-255.
[17] BICA C I D, BORSALI R, GEISSLER E, ROCHAS C. Dynamics of cellulose whiskers in agarose gels. 1: Polarized dynamic light scattering [J]. Macromolecules, 2001, 34: 5275-5279.
[18] BUTLER M F, GLASER N, WEAVER A C, KIRKLAND M, HEPPENSTALL-BUTLER M. Calcium carbonate crystallization in the presence of biopolymers [J]. Crys Growth Des, 2006, 6: 781-794.
[19] BUTLER M F, FRITH W J, RAWLINS C, WEAVER A C, HEPPENSTALL-BUTLER M. Hollow calcium carbonate microsphere formation in the presence of biopolymers and additives [J]. Crys Growth Des, 2009, 9: 534-545.
[20] FRICKE M, VOLKMER D, E.KRILL III C, KELLERMANN M, HIRSCH A. Vaterite polymorph switching controlled by surface charge density of an amphiphilic dendron-calix[4]arene [J]. Crys Growth Des, 2006, 6: 1120-1123.
[21] LOSTE E, D?AZ-MART? E, ZARBAKHSH A, C.MELDRUM F. Study of calcium carbonate precipitation under a series of fatty acid Langmuir monolayers using brewster angle microscopy [J]. Langmuir, 2003, 19: 2830-2837.
(Edited by YANG Bing)
Foundation item: Project supported by the Fundamental Research Funds for the Central Universities of China; Project (50573019) supported by the National Natural Science Foundation of China; Project (SKL2009-5) supported by Open Research Program of State Key Lab of Silicon Material, Zhejiang University, China
Received date: 2011-05-03; Accepted date: 2011-07-17
Corresponding author: YANG Xin-guo, Associate Professor, PhD; Tel: +86-731-88821610; Fax: +86-731-88823554; E-mail: xgyang@hnu.edu.cn