促植物生长内生细菌强化植物修复锌污染土壤
来源期刊:中国有色金属学报(英文版)2013年第8期
论文作者:龙新宪 陈雪梅 黄焕忠 卫泽斌 吴启堂
文章页码:2389 - 2396
关键词:内生菌;锌;生物有效性;植物修复;东南景天
Key words:endophytic bacteria; Zn; bioavailability; phytoremediation; Sedum alfredii
摘 要:以超积累植物东南景天为材料,采用盆栽试验探讨了接种内生菌VI8L2、II8L4和VI8R2对土壤Zn有效性、植物生长和吸收积累锌的影响。结果表明,接种内生菌VI8L2、II8L4和VI8R2能够促进东南景天在Zn污染土壤中的生长,其根系和地上部的生物量分别比对照增加了80%~525%和11%~47%。在人工ZnCO3污染土壤中,接种菌株VI8L2、II8L4和VI8R2显著增加了东南景天根系和地上部的Zn含量;在人工Zn3(PO4)2污染土壤中,菌株VI8L2使东南景天地上部和根系的Zn含量分别比对照增加了44%和39%,但是菌株Ⅳ8R2显著降低了东南景天地上部的Zn含量;在长期被酸性废水污染水稻土壤中,接种菌株VI8L2、II8L4和VI8R2显著增加了东南景天根系的Zn含量,但降低了地上部的Zn含量。这表明金属抗性促生细菌可用于强化植物修复重金属污染土壤。
Abstract: Three bacterial endophytes of Sedum alfredii, VI8L2, II8L4 and VI8R2, were examined for promoting soil Zn bioavailability and Zn accumulation in S. alfredii. Results showed that three strains were re-introduced into S. alfredii rhizosphere soils under Zn stress and resulted in better plant growth, as roots biomass increased from 80% to 525% and shoot biomass from 11% to 47% compared with the uninoculated ones. Strains IV8L2, II8L4 and IV8R2 significantly increased shoot and root Zn concentrations in the ZnCO3 contaminated soil. Inoculation with strain IV8L2 resulted in 44% and 39% higher shoot and root Zn concentrations, while strain IV8R2 significantly decreased shoot Zn concentration in the Zn3(PO4)2 contaminated soils. In the aged contaminated soil, isolates IV8L2, II8L4 and IV8R2 significantly increased root Zn concentration, but decreased shoot Zn concentration of Sedum alfredii. It suggested that endophytes might be used for enhancing phytoextraction efficiency.
Trans. Nonferrous Met. Soc. China 23(2013) 2389-2396
Xin-xian LONG1,2, Xue-mei CHEN1,2, Jonathan Woon-Chung WONG3, Ze-bin WEI1,2, Qi-tang WU1,2
1. College of Natural Science and Environment, South China Agricultural University, Guangzhou 510642, China;
2. Key Laboratory of Soil Environment and Waste Reuse in Agriculture of Guangdong Higher Education Institutes, South China Agricultural University, Guangzhou 510642, China;
3. Department of Biology, Hong Kong Baptist University, Kowloon Tong, Hong Kong SAR, China
Received 21 July 2012; accepted 27 November 2012
Abstract: Three bacterial endophytes of Sedum alfredii, VI8L2, II8L4 and VI8R2, were examined for promoting soil Zn bioavailability and Zn accumulation in S. alfredii. Results showed that three strains were re-introduced into S. alfredii rhizosphere soils under Zn stress and resulted in better plant growth, as roots biomass increased from 80% to 525% and shoot biomass from 11% to 47% compared with the uninoculated ones. Strains IV8L2, II8L4 and IV8R2 significantly increased shoot and root Zn concentrations in the ZnCO3 contaminated soil. Inoculation with strain IV8L2 resulted in 44% and 39% higher shoot and root Zn concentrations, while strain IV8R2 significantly decreased shoot Zn concentration in the Zn3(PO4)2 contaminated soils. In the aged contaminated soil, isolates IV8L2, II8L4 and IV8R2 significantly increased root Zn concentration, but decreased shoot Zn concentration of Sedum alfredii. It suggested that endophytes might be used for enhancing phytoextraction efficiency.
Key words: endophytic bacteria; Zn; bioavailability; phytoremediation; Sedum alfredii
1 Introduction
Zinc is the second most abundant transition metal in organisms after iron (Fe), and the only metal presented in all six enzyme classes (oxidoreductases, transferases, hydrolases, lyases, isomerases) [1]. Though Zn toxicity in crops is far less widespread than Zn deficiency, Zn toxicity occurs in soils contaminated by mining and smelting activities, in agricultural soils treated with sewage sludge, and in urban soils enriched by anthropogenic inputs of Zn [2]. Phytoextraction, using metal-accumulating plants to transport and concentrate heavy metal from the soil into their harvestable biomass, has been proposed as an environmentally friendly and low-input remediation technique [3,4]. Current literature suggests that metal bioavailability in soils is decisive for the success of phytoextraction in field [5]. In soils, metals exist as a variety of chemical species and the fraction of metals in soil solution, consisting of free hydrated ions, water-soluble organic and inorganic complexes and metals sorbed on dissolved organic matter are the most bioavailable form [6]. Unfortunately, low bioavailability is a major factor limiting phytoextraction efficiency in field, because only a very small portion of heavy metal is present in soil solution or exchangeable from soil colloids [7].
Heavy metal-resistant bacteria are ubiquitous in the environment, and their frequency is often increased in contaminated environment. Hyperaccumulators accumulate huge amounts of heavy metals and provide a specific environment for bacterial endophytes, which have to tolerate high concentrations of certain heavy metal [8]. Many metal-resistant endophytic bacteria have been isolated from various hyperaccumulators such as Alyssum bertolonii, Thlaspi caerulescens, Thlaspi goesingense and Nicotiana tabacum [8-11]. Bacteria have developed different heavy metal tolerance mechanisms involving exclusion, biosorption, precipitation or bioaccumulation both in external and intracellular spaces. These biochemical processes can influence the mobility and bioavailability of heavy metal in soils [12]. Some studies demonstrated that certain metal resistant bacteria increased heavy metal bioavailability through acidification, producing iron chelators, siderophores, organic acids, or mobilizing metal phosphates [13,14]. Besides their role in changing metal bioavailability, some bacterial endophytes can promote plant growth by similar mechanisms as plant growth promoting rhizobacteria (PGPR), including nitrogen fixation, phosphate solubilization, IAA production, and production of siderophore. Further, 1-aminocyclopropane-1-carboxylate (ACC) deaminase producing bacteria play an important role in the alleviation of heavy metal stress in plants [9,11,15,16]. Therefore, the efficiency of phytoextraction can be enhanced by careful application of such microbes possessing the ability of increasing soil metal bioavailability and promoting plant growth. In this study, the major aims of this study were to evaluate the ability of three Zn-mobilizing endophytic bacteria for enhancing plant growth and Zn uptakes in hyperaccumulator sedum aalfredii (S. alfredii) for improving the efficiency of phytoremediation of Zn-contaminated soils.
2 Experimental
2.1 Soil characterization and preparation
Two artificially Zn contaminated soils and one aged contaminated soil were used in this experiment. The artificially ZnCO3 and Zn3(PO4)2 contaminated soils were prepared as follows: A clean soil was collected from the farm of South China Agricultural University. The basic properties of the soil samples were pH (1:2.5 w/v water) 5.30, organic matter of 90.7 g/kg and total Zn of 74.3 mg/kg. Fine powder of ZnCO3 or Zn3(PO4)2 was mixed thoroughly with 10 kg soil to given 500 mg/kg of Zn and incubated at room temperature for 2 months for metal stabilization. The aged contaminated paddy soil was contaminated with Zn, Cd, Cu and Pb due to surface irrigation with the wastewater of Dabaoshan mine in Guangdong Province of China. The basic properties of the aged contaminated soil samples were pH (1:2.5 w/v water) 3.68, total Zn of 332 mg/kg, total Cd of 0.54 mg/kg, total Cu of 407 mg/kg and total Pb of 490 mg/kg. Soil samples were air-dried, ground, and passed through a 2 mm sieve for the bacterial solubilization of soil Zn experiment, or through a 5 mm-sieve for the pot experiment.
2.2 Bacterial strains and preparation of inoculums
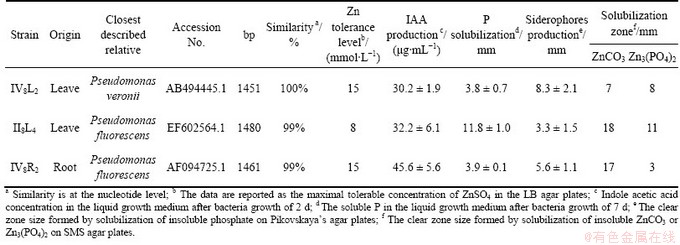
Endophytic bacteria VI8L2, II8L4 and VI8R2, identified as Pseudomonas veronii, Pseudomonas fluorescens and Pseudomonas fluorescens respectively, were isolated from the leaves and roots of Zn/Cd hyperaccumulator S. alfredii previously described by LONG et al [17]. Strains VI8L2, II8L4 and VI8R2 were the best at solubilizing ZnCO3 and Zn3(PO4)2 compound under in vitro condition. Strains VI8L2 and VI8R2 can tolerate up to 15 mmol/L Zn, and strain II8L4 can tolerate to 8 mmol/L Zn in LB solid medium. In addition, strains VI8L2, II8L4 and VI8R2 show plant growth promoting activity, such as IAA production, solubilization of Ca3(PO4)2 and production of siderophores (Table 1).
The bacteria were grown in LB broth in a shaking incubator at 30 °C and 160 r/min for 36 h. Immediately prior to inoculation, the bacterial culture was centrifuged at 5000 r/min and 4 °C for 10 min, the pelleted cell was washed with sterilized physiological saline three times. Bacterial inoculation was prepared by re-suspending the pelleted cells in sterile distilled water to get an inoculums density of about 3×108 mL-1.
2.3 Bacterial solubilization of soil Zn
Bacteria were cultured in SMS medium at 28 °C for 48 h, then centrifugated (10000 r/min) at 4 °C for 5 min. The supernatant including the bacterial metabolite was used to extract Zn from the three tested soils, using sterile SMS medium and deionized water as control. 10 mL cell supernatant, SMS medium or water was added to 2 g soils. Soil suspension was vibrated at 25 °C for 2 h, then centrifugated at 4000 r/min for 15 min. Zinc concentrations in the extracted solutions were determined by AAS.
Next, batch studies were carried out to evaluate the effect of bacterial inoculation on soil Zn availability. Bacterial inoculums of 0.8 mL (ca. 3×108 mL-1) were added to 2 g sterilized soils (steamed at 100 °C for 1 h on three consecutive days) in the 50 mL centrifugal tube, using sterilized water as a control. Three replicates were used for each treatment. All tubes were weighed and placed on an incubator at 30 °C. After 3 weeks, the tubes were again weighed to compensate for evaporation of water. 5 mL of 0.01 mol/L CaCl2 was added to each tube to extract the soil water-soluble (i.e. labile and bioavailable) Zn [18]. The soil suspensions were shaked at 200 r/min for 2 h, then centrifuged at 4000 r/min for 10 min and filtered. The concentration of Zn in the filtrate was determined by AAS.
Table 1 Endophytic bacteria used in this study
2.4 Influence of endophytic bacteria on S. alfredii growth and Zn uptake
Pot experiment was used to study the effects of the strains on plant growth and Zn uptake of S. alfredii using the above soils. Each pot contained 1.0 kg of sterilized soil (steamed at 100 °C for 1 h on three consecutive days). Three replicates were made for each treatment. Healthy and equal-sized stems of S. alfredii were surface sterilized with 0.1% (w/v) HgCl2 for 2 min, and were subsequently washed with sterilized deionized water 4 times. Two plants were transplanted to each pot.
For inoculation, bacterial suspensions (50 mL/pot) or sterile deionized water (as the control) were sprinkled on the soil surface for 10 d after transplanting. Plants were grown in a glass greenhouse under natural lighting and day/night temperature of 22/18 °C. The soil was moistened with sterilized deionized water and maintained at about 60% of the water holding capacity. After growth of 90 d, shoots were excised approximately 1 cm above the soil surface. The whole soil plus root system was placed onto a clean plastic sheet, and roots were carefully picked up manually. Shoots and roots were carefully washed with tap water and rinsed three times with deionized water. Growth parameters such as fresh mass and dry mass of the plants were measured. The contents of Zn, Fe, N and P in root and shoot tissues were also determined. Root and shoot Zn and Fe concentrations were determined by AAS after dry ashing at 550 °C. Total N and P in root and shoot were measured using automatic gerhardt kjeldahl determination device and vanadium molybdate yellow colorimetric method, respectively, after plant samples were digested with 5 mL concentrated H2SO4 and 1 mL H2O2. Soil pH was measured using a digital pH meter in a 1:2.5 suspension of soil-to-water ratio, and the available Zn concentrations in the rhizosphere soil were extracted with 0.01 mol/L CaCl2 and were analyzed by AAS.
2.5 Statistical analysis
All the values expressed were means ± S.D. (standard deviation) of the three replicates. Analysis of variance and the Student–Newman–Keuls test (P<0.05) were used to compare treatment means. All the statistical analyses were carried out using SAS 9.0.
3 Results
3.1 Effect of bacteria on soil Zn mobility
The metabolites of the three bacterial isolates strongly enhanced Zn extraction from soils (Fig. 1). The total Zn extracted from the ZnCO3 contaminated soils by the metabolites of strains VI8L2, II8L4 and VI8R2 were 206%, 141% and 94% higher than the sterile water, and were 134%, 89%, and 49% higher than the axenic SMS broth, respectively. The total Zn extracted from the Zn3(PO4)2 contaminated soils by the metabolites of strains VI8L2, II8L4 and VI8R2 were 229%, 134%, and 77% higher than the sterile water, and were 168%, 91%, and 45% higher than the axenic SMS broth, respectively. The total Zn extracted from the aged contaminated soil by the metabolites of strains IV8L2, II8L4 and IV8R2 increased by 43%, 29%, 9.3% compared with sterile water, and increased by 23%, 12%, 12% compared with the axenic SMS broth, respectively (Fig. 1).
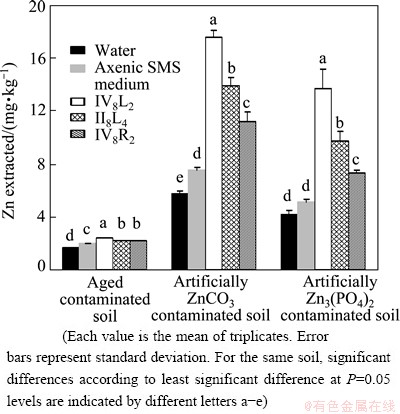
Fig. 1 Ability of bacterial metabolite to extract Zn from artificially ZnCO3 and Zn3(PO4)2 contaminated soils and aged contaminated soils
Re-inoculation strains IV8L2, II8L4 and IV8R2 to the ZnCO3 contaminated soils increased the CaCl2- extractable Zn concentrations by 51%, 14% and 81% compared with the control after 21 d, respectively. Strains IV8L2 and II8L4 significantly increased the CaCl2-extractable Zn concentrations in the Zn3(PO4)2 contaminated soils, while strain IV8R2 decreased the CaCl2-extractable Zn concentration. However, CaCl2- extractable Zn concentrations in the aged contaminated soils inoculated with strains IV8L2, II8L4 and IV8R2 were remarkably lower than the non-inoculated control (Table 2).
3.2 S. alfredii growth
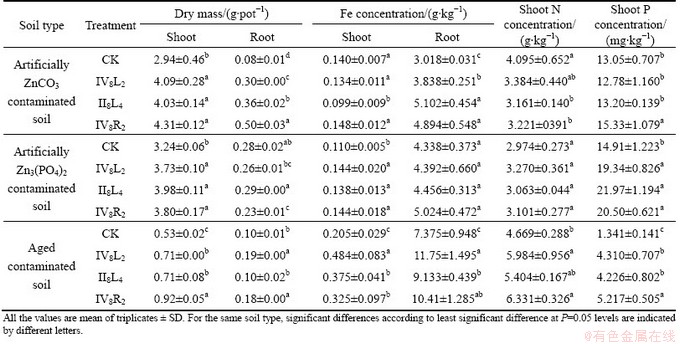
S. alfredii grew healthy in the ZnCO3 and Zn3(PO4)2 contaminated soils, but was severely inhibited in the aged contaminated soil due to adverse acid soil pH condition. Inoculation with strains IV8L2, II8L4 and IV8R2 all promoted the growth of S. alfredii in the aged contaminated soil and the ZnCO3 contaminated soil. Strain IV8R2 was the most effective strain for plant growth promotion, shoot and root biomasses of S. alfredii growing on the aged contaminated soils and the artificially ZnCO3 contaminated soils were 73%, 89% and 46%, 524% higher than the non-inoculated plants, respectively (Table 3). In the Zn3(PO4)2 contaminated soils, strains IV8L2, II8L4 and IV8R2 significantly increased shoot biomass, but did not stimulate root growth (Table 3).
The re-inoculation with strains IV8L2, II8L4 and IV8R2 affected Fe, P and N uptake by S. alfredii (Table 3). In the ZnCO3 contaminated soil, root Fe concentrations of S. alfredii inoculated with strains IV8L2, II8L4 and IV8R2 increased by 27%, 69% and 67% compared with the non-inoculated control, respectively. However, those strains decreased shoot N concentrations. In the Zn3(PO4)2 contaminated soils, strains IV8L2, IV8L4 and IV8R2 significantly increased shoot Fe and P concentrations, but had no significant effects on root Fe concentrations and shoot N concentrations. In the aged contaminated soils, shoot and root Fe concentrations, shoot N and P concentrations in S. alfredii inoculated with strains IV8L2, II8L4 and IV8R2 were all greatly higher compared with the control, strain IV8L2 was the best in stimulating Fe uptake and strain IV8R2 was the best in stimulating N and P uptake.
Table 2 Effects of bacterial inoculation on 0.01 mol/L CaCl2-extractable Zn concentration in soil
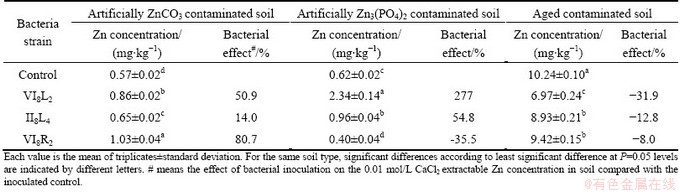
Table 3 Effects of inoculation with Zn solubilizing bacteria on shoot and root dry matter, Fe, N and P uptake by S. alfredii
3.3 Mobilization of soil Zn to S. alfredii
Re-inoculation strains IV8L2, II8L4 and IV8R2 to the ZnCO3 contaminated soils increased shoot Zn concentrations by 52%, 20% and 47% and root Zn concentrations by 85%, 64% and 74%, respectively, compared with the uninoculated plants. The maximum shoot (10.7 g/kg) and root Zn (2.4 g/kg) concentrations were observed in plant inoculated with strain IV8L2 (Fig. 2). In the Zn3(PO4)2 contaminated soils, shoot and root Zn concentrations in S. alfredi inoculated with strain IV8L2 were 45% and 39% higher than the control, respectively. Strain II8L4 had not great effect on shoot and root Zn concentration. However, strain IV8R2 significantly reduced shoot Zn concentration (Fig. 2). In the aged contaminated soils, strains IV8L2, II8L4 and IV8R2 significantly increased root Zn concentration by 82%, 99%, and 63%, respectively, but decreased shoot Zn concentrations.
In addition, in the artificially ZnCO3 contaminated soils inoculated with strains IV8L2, II8L4 and IV8R2, CaCl2-extractable Zn concentrations in rhizosphere soils of S. alfredi significantly increased, compared with the uninoculated control. This was associated with a drop of pH in the rhizosphere soil (Table 4). In the artificially Zn3(PO4)2 contaminated soils, inoculation with strains IV8L2, II8L4 and IV8R2 resulted in significant decrease of soil pH, isolate IV8L2 increased CaCl2-extractable Zn concentrations by 1.5-fold compared with the uninoculated rhizosphere soils, but isolate II8L4 or IV8R2 had no significant effects on CaCl2-extractable Zn concentrations (Table 4). In the aged contaminated soils, CaCl2-extractable Zn concentrations in the rhizosphere of S. alfredii inocubated with strains IV8L2, II8L4 and IV8R2 were greatly lower than that of the control, but there was no significant difference in pH between bacteria inoculated and uninoculated soils (Table 4).
4 Discussion
Recently, endophytic bacteria associated with heavy metal hyperaccumulators have attracted attention of several investigators due to their potential applications for assisting phytoremediation of heavy metal contaminated soil [9,18,19]. Pot experiment found that the inoculation of bacteria IV8L2, II8L4 and IV8R2 promoted the growth of S. alfredii, especially on the aged contaminated soil and the artificially ZnCO3 contaminated soil (Table 3). Generally, endophytic bacteria can benefit plant growth by phosphate solubilization, IAA or siderophore production, nitrogen fixation, and prevention of the growth or activity of plant pathogens [4,5]. The increase in plant growth caused by these three endophytic bacteria may be attributed to the production of IAA, increasing P and Fe supply, because strains IV8L2, II8L4 and IV8R2 all had the intrinsic ability of production of IAA and siderophore, and solubilization of phosphate (Table 1). Furthermore, pot experiment results proved that strains IV8L2, II8L4 and IV8R2 significantly enhanced the assimilation of Fe, P and N by S. alfredii. For example, strains IV8L2, II8L4 and IV8R2 increased root Fe concentrations of S. alfredii growing on the artificially ZnCO3 contaminated soil by 27%, 69% and 67%, respectively; shoot Fe and P concentrations in S. alfredii growing on the artificially Zn3(PO4)2 contaminated soils inoculated with strains IV8L2, II8L4 and IV8R2 were significantly higher than the non-inoculated plant; shoot and root Fe concentrations, shoot N and P concentrations in S. alfredii inoculated with strains IV8L2, II8L4 and IV8R2 were greatly higher than that of the control, when grown on the extremely acid aged contaminated soils (Table 3). This result is in agreement with other studies, which have also proved that plant growth promoting rhizosphere or endophytic bacteria can assist plant establishment on contaminated soils by improving nutrient uptake by plant. For example, BARZANTI et al [10] reported that 83% of bacterial isolates recovered from Alyssum bertolonii could produce siderophores and promote the plant growth under Ni stress. The inoculation with metal-resistant bacterial Bacillus weihenstephanensis SM3 increased the fresh mass and dry mass of Helianthus annuus by 47% and 23% in Ni contaminated soil and by 35% and 16% in Cu contaminated soil, respectively, compared with non-inoculated plants [4]. The increase in plant growth caused by strain SM3 may be attributed to the solubilization of phosphate and production of IAA. SHENG et al [14] reported that Pb-resistant endophytic bacteria Pseudomonas fluorescens G10 and Microbacterium sp. G1, which can produce IAA and siderophores, not only increased root elongation of inoculated rape seedlings, but also increased root dry mass by 23%-37% and shoot dry mass by 12%-29%.
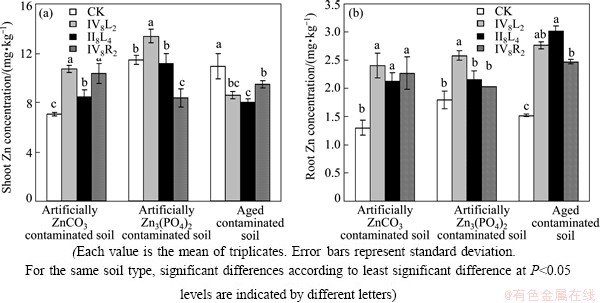
Fig. 2 Effects of inoculation with Zn solubilizing bacteria on Zn uptake by S. alfredii
Table 4 Effects of bacterial inoculation on soil pH and 0.01 mol/L 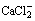 extractable Zn concentration in rhizosphere soils of Sedum alfredii
extractable Zn concentration in rhizosphere soils of Sedum alfredii
Low bioavailability of soil metals is one limiting factor for the success of phytoextraction in field [5]. Soil metal bioavailability is mediated by many interacting factors associated with soil properties, metal characteristics and effects of plant roots and the associated microbial community [8]. Although the uptake and accumulation of metals by plants can be enhanced by addition of chemical chelates, such as EDTA, EDDS, TNT, EDGA and citric acid, these expensive compounds can increase the metal-leaching risk and impart negative effects on plant growth or soil structure [20]. Certain soil microorganisms can increase solubility and change speciation of metals through producing organic ligands, exudating metabolites (e.g. organic acids, microbial siderophores), reducing soil pH, and/or solubilizing metal-phosphates [21]. The present study shows that the bacterial metabolites of strains IV8L2, II8L4 and VI8R2 extracted much higher Zn from the artificially ZnCO3 and Zn3(PO4)2 contaminated soils and the aged contaminated soils than those extracted by axenic SMS broth and water (Fig. 1). Production of H+ and organic acids by rhizosphere organisms appear to be the most significant mechanism for metal mobilization. For example, SARAVANAN et al [13] reported the production of 5-ketogluconic acid, a major gluconic acid derivative product that aids the solubilization of different Zn compounds by endophyte G. diazotrophicus under in vitro conditions. MAJEWSKA et al [22] found that increases in microbially-produced citric acid, acetic acid, catechol siderophores, and Fe-chelators may have contributed to cadmium mobilization within soils, decreasing the pH from 6.5 to 5 after 48 h. The production of oxalic acid, tartaric acid, formic acid and acetic acid had a significant correlation (P<0.01) with the concentrations of Cd and Zn mobilized from CdCO3 and ZnO by rhizosphere bacteria associated with a Cd/Zn hyperaccumulator S. alfredii [21].
We also found that the CaCl2-extractable Zn concentration in the artificially ZnCO3 contaminated soil inoculated with isolates IV8L2, II8L4 and IV8R2 was significant higher than that of the uninoculated control, and inoculation with strains IV8L2 significantly increased the CaCl2-extractable Zn concentration in the artificially Zn3(PO4)2 contaminated soil (Table 2, Table 4). These observations are in agreement with other research works. For example, ABOU-SHANAB et al [23] reported that the concentration of extractable Ni was increased from a high-Ni soil of 2.2-2.6 mg/kg when the soil was inoculated with Microbacterium arabinogalactanolyticum AY509224, which has the ability of producing acid and siderophore and solubilizing inorganic phosphate. These results indicate that the activity of soil bacteria would likely have a significant effect on increasing the bioavailability of metals in soils. In the pot experiment study, we also found that inoculation with strains IV8L2, II8L4 and IV8R2 increased shoot Zn concentrations by 20%-52% and root Zn concentrations by 64%-85% in S. alfredii grown on the artificially ZnCO3 contaminated soils, respectively; strain Ⅳ8L2 increased shoot concentration by 45% and root Zn concentration by 39% in S. alfredi grown on the artificially Zn3(PO4)2 contaminated soil; strains IV8L2, II8L4 and IV8R2 increased root Zn concentration of S. alfredii grown on the Dabaoshan contaminated soil by 63%-99% (Fig. 3). These promoting heavy metal accumulation effects of bacterial inoculation were reported also by other scientists. For example, WHITING et al [24] reported that the addition of a mixed inoculum of Microbacterium saperdae, Pseudomonas monteilii and Enterobacer cancerogenes to surface-sterilized seeds of Thalaspi caerulescens increased the Zn concentration in shoots 2-fold and total Zn accumulation 4-fold compared with non-inoculated controls, respectively. Four Zn-tolerant bacteria (Bacillus subtilis, B. cereus, Flavobacterium sp. and Pseudomonas aeruginosa) significantly increased Zn concentrations in the roots and shoots of O. violaceus plants compared with non-inoculated plants [25].
However, we found that the uninoculated and inoculated S. alfredii all grew very poor on the acid aged contaminated soil, even though the bacterial inoculation with strains IV8L2, II8L4 and IV8R2 increased shoot and root biomass of S. alfredii. Mining wastewater polluted soils are often difficult for plant establishment and growth due to a combination of factors including metal toxicity, acidic pH and stressed microbial communities [26]. Therefore, how to remediate such acidic heavy metal contaminated soils by combining chemical and bioremediation needs further to investigate.
5 Conclusions
Endophytic bacteria, including IV8L2, II8L4 and IV8R2, can increase soil Zn bioavailability, and their intrinsic property of plant growth promoting might make them one of the most suitable choice for improving phytoremediation of Zn contaminated soils.
References
[1] BROADLEY M R, WHITE P J, HAMMOND J P, ZELKO I, LUX A. Zinc in plants [J]. New Phytologist, 2007, 173: 677-702.
[2] CHANEY R L. Zinc phytotoxicity [C]//ROBSON A D. Zinc in soil and plants [M]. Dordrecht: the Netherlands: Kluwer Academic Publishers, 1993: 135-150.
[3] MCGRATH S P, ZHAO F J. Phytoextraction of metals and metalloids from contaminated soils [J]. Curr Opin Biotechnol, 2003, 14: 277-282.
[4] CHANEY R L, MALIK M, LI Y M, BROWN S L, BREWER E P, ANGLE J S, BAKER A J M. Phytoremediation of soil metals [J]. Curr Opin Biotechnol, 1997, 8: 279-284.
[5] WENZEL W M. Rhizosphere processes and management in plant-assisted bioremediation (phytoremediation) of soils [J]. Plant Soil, 2009, 321: 385-408.
[6] GLICK B R. Using soil bacteria to facilitate phytoremediation [J]. Biotechnology Advance, 2010, 41: 109-117.
[7]  D. Enhanced heavy metal phytoextraction [C]// MACKOVA M, et al. Phytoremediation and Rhizoremediation. Dordrecht: the Netherlands: Springer, 2006: 115-132.
D. Enhanced heavy metal phytoextraction [C]// MACKOVA M, et al. Phytoremediation and Rhizoremediation. Dordrecht: the Netherlands: Springer, 2006: 115-132.
[8] SESSITSCH A, PUSCHENREITER M. Endophytes and rhizosphere bacteria of plants growing in heavy metal-containing soils [C]//DION P, NAUTIYAL C S. Microbiology of Extreme Soils, Soil Biology. Berlin, Heidelberg: Springer-Verlag, 2008: 317-332.
[9] IDRIS R, TRIFONOVA R, PUSCHENREITER M, WENZEL W W, SESSITSCH A. Bacterial communities associated with flowering plants of the Ni hyperaccumulator Thlaspi goesingense [J]. Applied and Environmental Microbiology, 2004, 70: 2667-2677.
[10] BARZANTI R, OZINO F, BAZZICALUPO M, GABBRIELLI R, GALARDI F, GONNELLI C, MENGONI A. Isolation and characterization of endophytic bacteria from the nickel hyperaccumulator plant Alyssum bertolonii [J]. Microbiology Ecology, 2007, 53: 306-316.
[11] MASTRETTA C, TAGHAVI S, VAN DER LELIE D, MENGONI A, GALARDI F, GONNELLI C, BARAC T, BOULET J, WEYENS N, VANGRONSVELD J. Endophytic bacteria from seeds of Nicotiana tabacum can reduce cadmium phytotoxicity [J]. International Journal of Phytoremediation, 2009, 11: 251-267.
[12] HAFERBURG G, KOTHE E. Microbes and metals: Interactions in the environment [J]. Journal Basic Microbiology, 2007, 47: 453-467.
[13] SARAVANAN V S, KALAIARASAN P, MADHAIYAN M, THANGARJU M. Solubilization of insoluble zinc compounds by Gluconacetobacter diazotrophicus and the detrimental action of zinc ion (Zn2+) and zinc chelates on root knot nematode meloidogyne incognita [J]. Letters in Applied Microbiology, 2007, 44: 235-241.
[14] SHENG X F, HE L Y, WANG Q, YE H, JIANG C. Effects of inoculation of biosurfactant-producing Bacillus sp. J119 on plant growth and cadmium uptake in a cadmium-amended soil [J]. Journal of Hazardous Materials, 2008, 155: 17-22.
[15] HARDOIM P R, van OVERBEEK L S, van ELSAS J D. Properties of bacterial endophytes and their proposed role in plant growth [J]. Trends Microbiology, 2008, 16: 467-471.
[16] KUKLINSKY-SOBRAL J, ARAUJO W L, MENDES R, GERALDI I O, PIZZIRANI-KLEINER A A, AZEVEDO J L. Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion [J]. Environment Microbiology, 2004, 6: 1244-1251.
[17] LONG X X, CHEN X M, CHEN Y G, WONG J W, WEI Z B, WU Q T. Isolation and characterization endophytic bacteria from Sedum alfredii Hance and their potential to promote phytoextraction of zinc polluted soil [J]. World Journal Microbiology Biotechnology, 2010, 27: 1197-1207.
[18] HE L Y, CHEN A J, REN G D, ZHANG Y F, QIAN M, SHENG X F. Increased cadmium and lead uptake of a cadmium hyperaccumulator tomato by cadmium-resistant bacteria [J]. Ecotoxicology and Environmental Safety, 2009, 72: 1343-1348.
[19] NEWMAN L A, REYNOLDS C M. Bacteria and phytoremediation: new uses for endophytic bacteria in plants [J]. Trends in Biotechnology, 2005, 23: 6-8.
[20]  P, BOUWMAN L, JAPENGA J, DRAAISMA C. Potentials and drawbacks of chelate-enhanced phytoremediation of soils [J]. Environmental Pollution, 2002, 116: 109-121.
P, BOUWMAN L, JAPENGA J, DRAAISMA C. Potentials and drawbacks of chelate-enhanced phytoremediation of soils [J]. Environmental Pollution, 2002, 116: 109-121.
[21] LI W C, YE Z H, WONG M H. Metal mobilization and production of short-chain organic acids by rhizosphere bacteria associated with a Cd/Zn hyperaccumulating plant Sedum alfredii [J]. Plant Soil, 2010, 326: 453-467.
[22] MAJEWSKA M, KUREK E, ROGALSKI J. Microbially mediated cadmium sorption/desorption processes in soil amended with sewage sludge [J]. Chemosphere, 2007, 67: 724-730.
[23] ABOU-SHANAB R A I, DELORME T A, ANGLE J S, CHANEY R L, GHANEM K, MOAWAD H, GHOZLAN H A. Phenotypic characterization of microbes in the rhizosphere of Alyssum murale [J]. International Journal of Phytoremediation, 2003, 5: 367-379.
[24] WHITING N S, DE SOUZA P M, TERRY N. Rhizosphere bacteria mobilize Zn for hyperaccumulation by Thlaspi caerulescens [J]. Environment Science and Technology, 2001, 35: 3144-3150.
[25] HE C Q, TAN G E, LIANG X, DU W, CHEN Y L, ZHI G Y, ZHU Y. Effect of Zn-tolerant bacterial strains on growth and Zn accumulation in Orychophragmus violaceus [J]. Applied Soil Ecology, 2010, 44: 1-5.
[26] MENDEZ M O, MAIER R M. Phytostabilization of mine tailings in arid and semiarid environments-An emerging remediation technology [J]. Environ Health Perspect, 2008, 116: 278-283.
龙新宪1,2,陈雪梅1,2,黄焕忠3,卫泽斌1,2,吴启堂1,2
1. 华南农业大学 资源环境学院,广州 510642;
2. 华南农业大学 广东省教育厅土壤环境与废物利用重点实验室,广州 510642;
3. 香港浸会大学 生物系,香港
摘 要:以超积累植物东南景天为材料,采用盆栽试验探讨了接种内生菌VI8L2、II8L4和VI8R2对土壤Zn有效性、植物生长和吸收积累锌的影响。结果表明,接种内生菌VI8L2、II8L4和VI8R2能够促进东南景天在Zn污染土壤中的生长,其根系和地上部的生物量分别比对照增加了80%~525%和11%~47%。在人工ZnCO3污染土壤中,接种菌株VI8L2、II8L4和VI8R2显著增加了东南景天根系和地上部的Zn含量;在人工Zn3(PO4)2污染土壤中,菌株VI8L2使东南景天地上部和根系的Zn含量分别比对照增加了44%和39%,但是菌株Ⅳ8R2显著降低了东南景天地上部的Zn含量;在长期被酸性废水污染水稻土壤中,接种菌株VI8L2、II8L4和VI8R2显著增加了东南景天根系的Zn含量,但降低了地上部的Zn含量。这表明金属抗性促生细菌可用于强化植物修复重金属污染土壤。
关键词:内生菌;锌;生物有效性;植物修复;东南景天
(Edited by Hua YANG)
Foundation item: Project (40973055) supported by the National Natural Science Foundation of China; Project (U0833004) supported by the NSFC-Guangdong Joint Foundation of China
Corresponding author: Xin-xian LONG; Tel/Fax: +86-20-85288326; E-mail: longxx@scau.edu.cn
DOI: 10.1016/S1003-6326(13)62746-6

