J. Cent. South Univ. Technol. (2009) 16: 0541-0545
DOI: 10.1007/s11771-009-0090-7

Influence of surface finish and annealing treatment on
oxidation behavior of Ti-48Al-8Cr-2Ag alloy
XI Yan-jun(席艳君), LIU Yong-jun(刘泳俊)
(School of Materials and Chemical Engineering, Zhongyuan University of Technology, Zhengzhou 450007, China)
Abstract: The effect of surface finish and annealing treatment on the oxidation behavior of Ti-48Al-8Cr-2Ag (molar fraction, %) alloy was investigated at 900 and 1 000 ℃, respectively in air. Thermal gravimetric analysis (TGA) was conducted for the characterization of oxidation kinetics. The microstructures of oxide scales were studied by scanning electron microscopy (SEM) and transmission election microscopy (TEM) techniques. Unfavorable effect of the annealing treatment on the oxidation behavior of the coating was also investigated. The results indicate that the oxidation behavior of the alloy is influenced by surface finish and annealing treatment. The oxidation rate of ground sample is lower than that of the polished alloy at 1 000 ℃ in air. The former forms a scale of merely Al2O3, and the latter forms a scale of the mixture of Al2O3 and TiO2. Annealing can improve the formation of TiO2.
Key words: Ti-48Al-8Cr-2Ag alloy; coating; surface finish; annealing; oxidation behavior
1 Introduction
Conventional TiAl alloys do not form protective oxide scales during high temperature exposure [1-5]. TiAl-base intermetallics have received more attention as potential high temperature structural materials because they have many properties beneficial to high temperature applications, including low density, good high temperature strength, and high stiffness. In order to enhance the oxidation resistance of TiAl-base intermetallics, several kinds of coating have been investigated [6-9]. Recently, some promising results have been found in the Ti-Al-Cr system [10-16]. However, there have been few reports concerning the influence of surface finish and annealing treatment on the oxidation behavior of TiAl alloy.
Among coating processes, magnetron sputtering is a very suitable method to manufacture thin films with complex and homogeneous chemical composition as well as uniform morphology and coating thickness. The deposition process runs in a vacuum chamber refilled with high purity argon. When no external heating is used during the coating deposition, substrate temperatures are comparatively low due to impinging species and argon ions as well as latent heat of condensation. Therefore, the microstructure change of the substrate alloy can be avoided during deposition, which is sometimes a problem for plasma spraying and evaporation techniques on heat sensitive materials [17].
Some researchers studied the oxidation of TiAl alloy and its coating [4-5, 8, 12], but few investigated the influence of the surface treatment on the oxidation behavior. In this work, a microcrystalline TiAl coating was deposited on the surface of the same substrate by magnetron sputtering. The oxidation behavior of TiAl alloy and its coating was investigated. Special emphasis was placed on a detailed examination of the effect of surface finish and annealing treatment on the oxidation behavior of Ti-48Al-8Cr-2Ag (molar fraction, %) alloy and coating at 900 and 1 000 ℃ in air.
2 Experimental
The nominal composition of the alloy was Ti-48Al-8Cr-2Ag. The resulting microstructure was γ-TiAl and Laves phase [12]. The Ti-48Al-8Cr-2Ag coating was prepared by magnetron sputtering technique. The target and the specimens, with dimensions of 380 mm×126 mm×7 mm and 10.0 mm×10.0 mm×2.5 mm respectively, were cut from an alloy ingot. The specimens were ground 600-grit SiC paper, peened and ultrasonically cleaned in ethanol. The peening treatment was performed to improve the adhesive energy. The morphology and microstructure of the coating were narrated in detail elsewhere [12]. The sputtering parameters were as follows: the pressure of reaction chamber, 0.5×10-2 Pa; argon pressure, 0.2-0.3 Pa; substrate temperature, 200 ℃; power, 1 800 W; and sputtering time, 8 h. The chemical composition of the
coating detected was Ti-49.29Al- 7.34Cr-1.38Ag (molar fraction, %). The surface of the coating showed the nodular morphology, and the cross exhibited columnar crystal. The thickness of the coating was almost 20 mm. The coating was annealed at 900 ℃ for 2 h.
Thermal gravimetric analysis (TGA) was conducted for the characterization of oxidation kinetics by measuring continuous mass changes. All specimens were oxidized isothermally. Different experiments were carried out to separate the effects of surface finish and annealing. The alloys were cut and ground through 600-grit SiC paper or polished down to a 1 μm diamond finish. Oxidation experiments were then carried out in air. The oxidized samples were characterized by SEM and TEM techniques.
3 Results and discussion
3.1 Effect of surface finish on oxidation behavior on Ti-48Al-8Cr-2Ag alloy
The TGA measurement of Fig.1 shows the influence of surface finish on the oxidation behavior of the Ti-48Al-8Cr-2Ag alloy. The kinetics for oxidation in air depends on the surface finish of the specimens after exposure. The ground specimen (600-grit SiC) oxidizes much more slowly compared to the specimen polished with 1 μm diamond paste.
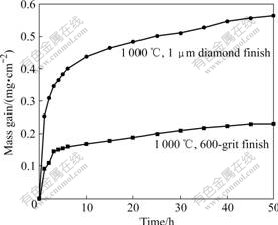
Fig.1 TGA data for isothermal oxidation in air showing influence of surface finish on oxidation of TiAl alloy
Fig.2 shows the cross sectional microstructure of the oxide scale at 1 000 ℃ in air. After oxidation for 50 h, a dense Al2O3 scale forms on the ground specimen with thickness of 4 mm without any TiO2 found. An Al-depleted layer is found obviously. The formation of oxide scale leads to a depleted region beneath the scale. By TEM investigation, we found that a layer beneath the scale consisted of Z-phase without α2-Ti3Al phase found [18]. While an oxide scale with a thickness of 12 mm forms on the polished specimen after 50 h oxidation, as

Fig.2 Cross sectional microstructures of ground sample (a) and polished specimen (b) after oxidation at 1 000 ℃ for 50 h in air
shown in Fig.2(b). The inner part of the scale is mainly composed of a dense Al2O3 layer. An Al-depleted layer near the substrate forms. And the outer layer is TiO2 with the thickness about 7 mm. The scale is adherent to the substrate.
3.2 Effect of annealing on oxidation behavior of Ti-48Al-8Cr-2Ag coating
Fig.3(a) shows XRD pattern of the sputtering Ti-48Al-8Cr-2Ag coating. The non-equilibrium coating is caused by the low temperature sputtering process that cannot provide sufficient mobility of the condensing species during coating growth, thus freezing impinging particles are at those sites where they first hit the substrate. The broad peaks reflect crystallographically fine structures that are obviously not perfectly crystallized, as can also be concluded from the absence of other peaks.
Fig.3(b) shows the XRD pattern of TiAl alloy with Ti-48Al-8Cr-2Ag coating after exposure at 900 ℃ for 2 h. The phase transforms from the metastable as-coated one to a more stable microstructure in the sense of thermodynamic stability. The coating shows peaks for the γ-TiAl and Laves phases.
An isothermal oxidation test was performed at 900 and 1 000 ℃ for 50 h to investigate the oxidation behavior of TiAl alloy coated with Ti-48Al-8Cr-2Ag alloy. Fig.4 shows the isothermal oxidation kinetics of Ti-48Al-8Cr-2Ag coating and annealed Ti-48Al-8Cr-2Ag
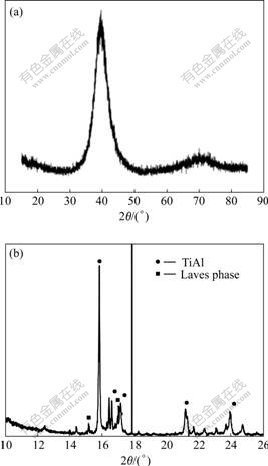
Fig.3 XRD patterns of sputtering Ti-48Al-8Cr-2Ag alloy (a) and as coated/annealed Ti-48Al-8Cr-2Ag alloy (b)

Fig.4 Oxidation kinetic curves of as-coated Ti-48Al-8Cr-2Ag and as coated/annealed Ti-48Al-8Cr-2Ag coatings
coating. The coating exhibits a very low oxidation rate at 900 and 1 000 ℃, and oxidation kinetics accords with parabolic law. But after annealing, the coating exhibits a much higher mass gain at the same temperature. All the samples show similar oxidation kinetics with an initial protective parabolic behavior. It is interesting that the as-coated/annealed Ti-48Al-8Cr-2Ag alloys change to faster mass gain kinetics after 10 h. This fact proves the unfavorable effect of the annealing treatment on the original structure on the sample surface.
The oxide scale forms on as-coated Ti-48Al-8Cr- 2Ag alloy, as shown in Figs.5-7. Fig.5 exhibits the oxide scale formed on the coating at 900 and 1 000 ℃. A scale of merely Al2O3 forms on the coating, which exhibits the excellent oxidation resistance.
The oxide scale on the as-coated/annealed coating during air exposure contains TiO2 and α-Al2O3, as shown in Figs.6 and 7. Some areas with TiO2 interpret the high oxidation rate of the annealed coating at 900 ℃. At 1 000 ℃, a layer of fine-grain oxide forms beneath a few dispersed oxide nodules. EDX spectra of these oxides indicate that a continuous Al2O3 scale with occasional TiO2 nodules forms on the alloy. The oxide nodules are composed of well-crystallized crystals and have a porous feature. The cross sectional microstructure corresponding to TiO2 nodules indicates a scale of mixture of TiO2 and Al2O3. It should be noted that serious oxidation occurs near the suspending pore after oxidation at 1 000 ℃ for 50 h, as shown in Fig.7(b), which is one of the reasons for the high mass gain for as coated/annealed alloy.
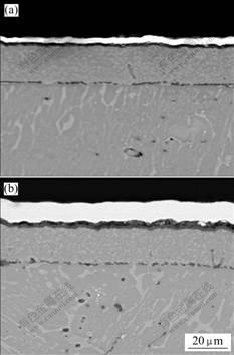
Fig.5 Cross sectional microstructures of as-coated Ti-48Al- 8Cr-2Ag alloy after oxidation for 50 h at 900 ℃ (a) and 1 000 ℃ (b)

Fig.6 Cross sectional microstructure of as-coated/annealed Ti-48Al-8Cr-2Ag alloy after oxidation at 900 ℃ for 50 h
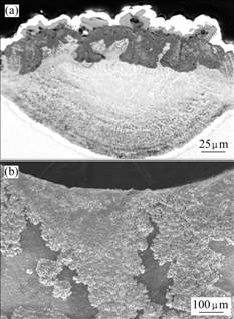
Fig.7 SEM images of as-coated/annealed Ti-48Al-8Cr-2Ag alloy after oxidation at 1 000 ℃ for 50 h: (a) Cross sectional microstructure corresponding to TiO2 nodules; (b) Surface morphology near suspending pore
3.3 Discussion
The experimental results for oxidation of the alloy in air indicate that the surface treatment influences the oxidation behavior significantly, as shown in the TGA measurement of Fig.1. This effect was also observed by DETTENWANGER et al [5] and was related to the homogenization of the alloy surface resulted from the grinding process. The surface finish with 1 mm diamond results in two to three orders of magnitude faster oxidation rate compared with ground specimens. The results are the same as those from DETTENWANGER et al [5]. DETTENWANGER et al [5] found that the oxide scale formed on the polished specimen consisted of a mixture of Al2O3 and TiO2, while a continuous alumina layer formed on the ground specimen. In addition, the depleted layer was also present on the ground sample after exposure. They found that the grinding surface resulted in the formation of thick recrystallization layer on the metal surface and no further growth of the zone into the base metal occurred after longer time. The surface damage introduced by the grinding process is much greater than that by the polishing procedure because the deformation zone near the surface of the alloy diminishes at the beginning of the exposure due to the recrystallization process [2]. Due to the higher defect density, an increase in diffusivity through short circuit diffusion paths can increase the diffusivity of Al. The formation of a continuous Al2O3 scale leads to the significantly better oxidation resistance.
The surface finish effect is not sufficient for the formation of a continuous Al2O3 scale. The addition of Cr and Ag into the TiAl alloy is another reason for the formation of Al2O3 scale. PERKINS and MEIER [19] found that the Cr effect on the oxidation resistance of TiAl alloy was related to the Laves phase, which increased the Al diffusion coefficient and decreased the solubility of N and O, thus decreasing the critical Al content needed for the Al2O3 scale formation. BRADY et al [13-14] studied the role of Cr in promoting the formation of protective Al2O3 scale in γ-based Ti-Al-Cr alloys. Laves phase not only increased the activity ratio a(Al)/a(Ti), but also possessed low oxygen permeability [14]. Hence, the selective oxidation of Al was promoted. Stabilization of Z phase was one of the most important factors for excellent oxidation resistance of Ti-48Al-8Cr-2Ag alloy in air. Hence, the Ti-48Al-8Cr-2Ag alloy exhibited excellent oxidation resistance at 1 000 ℃ partly because of the reasons mentioned above. In addition, the homogenization of the chemical composition on the ground surface can also significantly influence the scale formation.
From the oxidation kinetics (Fig.4), we can see that all the samples show similar oxidation kinetics with an initial protective parabolic behavior. But the as-coated/ annealed TiAl alloy changes to faster mass gain kinetics after oxidation for 10 h. This fact proves the unfavorable effect of the annealing treatment on the original structure on the surface of the sample. After being annealed at 900 ℃, the phase is transformed from the metastable as-coated to a more stable microstructure in the sense of thermodynamic stability and crystal grain increases with elevating temperature. When as-coated/ annealed TiAl alloy is exposed to air at high temperature, Ti diffusion rate increases and some TiO2 nodules form, thus oxidation rate increases. When original Al2O3 scale is destroyed, the oxidation kinetics changes from protective parabolic behavior to faster mass gain kinetics.
4 Conclusions
(1) The oxidation behavior of Ti-48Al-8Cr-2Ag alloy is influenced by surface finish and annealing treatment.
(2) Grinding process increases the defect density, providing short circuit diffusion paths for Al external diffusion. Therefore, the ground specimen shows a slower oxidation rate than the polished sample.
(3) Annealing treatment changes the microstructure of the coating on the surface of the sample. Therefore, unfavorable effect of the annealing treatment on oxidation is investigated.
References
[1] ZHU Ming, LI Mei-shuan, DUO Shu-wang, ZHOU Yan-chun. Improvement on the oxidation resistance of a Ti3Al based alloy by Cr1-xAlxN (0≤x≤0.47) coating [J]. J Mater Sci Technol A, 2007, A23(3): 373-379.
[2] FUKUMOTO M, HARA M, NAGATAKI T. Effect of Al electrodeposition treatment using molten salt on the high-temperature oxidation of TiAl [J]. Oxidation of Metals, 2004, 61(1/2): 1-6.
[3] XIONG Yu-ming, ZHU Sheng-long, WANG Fu-hui, LEE Chang-hee. Effect of vitreous enamel coating on the oxidation behavior of Ti6Al4V and TiAl alloys at high temperatures [J]. J Coat Technol Res, 2008, 5(1): 93-98.
[4] BECKER S, RAHMEL A, SCHORR M, SCHUTZE M. Mechanism of isothermal oxidation of the intermetallic TiAl and of TiAl alloys [J]. Oxid Met, 1992, 38: 425-429.
[5] DETTENWANGER F, SCHUMANN E, RUHLE M, RAKOWSKI J J, MERIER G H. Microstructure study of oxidized γ-TiAl [J]. Oxid Met, 1998, 50: 269-275.
[6] XIANG Z D, ROSE S R, DATTA P K. Codeposition of Al and Si to form oxidation-resistant coatings on TiAl by the pack cementation process [J]. Mater Chem Phi, 2003, 80: 482-489.
[7] NISHIMOTO T, IZUMI T, SHIGENARI, SHIGENARI, NATITA T. Two-step Cr and Al diffusion coating on TiAl at high temperatures [J]. Intermetallics, 2003, 11: 225-235.
[8] NISHIMOTO T, IZUMI T, SHIGENARI, SHIGENARI, NATITA T. Effect of coating layer structures and surface treatments on the oxidation behavior of a Ti-50 at.%Al alloy [J]. Intermetallics, 2003, 11: 459-466.
[9] MARIE-PIERRE B, BENOIT G, PIEERE J, CATHERINE R. MCrAlY coating developed via a new electroless-like route: Influence of deposition parameters [J]. Surf Coat Technol, 2003, 162: 248-260.
[10] WANG F, LOU H, WU W. The oxidation resistance of a sputtered microcrystalline TiAl intermetallic-compound film [J]. Oxid Met 1995, 43: 395-409.
[11] TANG Z, WANG F, WU W. Effect of a sputtered TiAlCr coating on the oxidation resistance of TiAl intermetallic compound [J]. Oxid Met, 1997, 48: 511-525.
[12] XI Yan-jun, WANG Fu-hui, HE Lian-long. Oxidation and hot-corrosion behavior of sputtered Ti-48Al-8Cr-2Ag nanocrystalline coating [J]. Mater Sci Forum, 2004, 461: 1181-1187.
[13] BRADY M P, SMIALEK J L, SMITH J. The role of Cr promoting protective aluminia scale formation by γ-based Ti-Al-Cr alloys: Compatibility with alumina and oxidation behavior in oxygen [J]. Acta Metall Meter, 1997, 45: 2357-2369.
[14] BRADY M P, SMIALEK J L, HUMPHRAY D L. The role of Cr in promotion protective alumina scale formation by γ-based Ti-Al-Cr alloys—Oxidation behavior in air [J]. Acta Metall Meter, 1997, 45: 2371-2382.
[15] TANG Z, SHEMET V, NIEWOLAK L, QUADAKKERS W J. Intermetallics: Effect of Cr addition on oxidation behavior of Ti-48Al-2Ag alloys [J]. Intermetallics, 2003, 11: 1-8.
[16] MURRIS I, JACOB Y P, HAANAPPEL V A C, STROOSNIJDER M F. High-temperature oxidation behavior of chromium: Effect of different batches [J]. Oxid Met, 2001, 55: 307-331.
[17] LEYENS C, SCHMIDT M, PETERS M, KAYSSER W A. Sputtered intermetallic Ti-Al-X coatings: Phase formation and oxidation behavior [J]. Meter Sci Eng A, 1997, 239/240: 680-687.
[18] XI Yan-jun, LU Wei, GUO Chang-you, HE Lian-long, WANG Fu-hui. Microstructure of oxide scale formed on Ti-48Al-8Cr-2Ag alloy in air at 900-1 000 ℃ [J]. Oxid Met, 2005, 63(3): 229-239.
[19] PERKINS R A, CHIANG KT, MEIER G H. Formation of alumina on Ti-Al alloys [J]. Scripta Metallurgica, 1987, 21 (11): 1505-1510.
(Edited by CHEN Wei-ping)
Foundation item: Project(2007430028) supported by the Science and Technique Foundation of Henan Educational Committee, China
Received date: 2008-10-28; Accepted date: 2009-02-22
Corresponding author: XI Yan-jun, PhD; Tel: +86-13592543516; E-mail: yjxi@sina.com