J. Cent. South Univ. (2013) 20: 1151-1155
DOI: 10.1007/s11771-013-1597-5
Preparation and effects of W-doping on electrochemical properties of spinel Li4Ti5O12 as anode material for lithium ion battery
ZHANG Xin-long(张新龙), HU Guo-rong(胡国荣), PENG Zhong-dong(彭忠东)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2013
Central South University Press and Springer-Verlag Berlin Heidelberg 2013
Abstract: W-doped Li4Ti5O12 in the form of Li4Ti4.95W0.05O12 was firstly synthesized via solid state reaction. X-ray diffraction (XRD) and scanning electron microscope (SEM) were employed to characterize the structure and morphology of Li4Ti4.95W0.05O12. W-doping does not change the phase composition and particle morphology, while remarkably improves its cycling stability at high charge/discharge rate. Li4Ti4.95W0.05O12 exhibits an excellent rate capability with a reversible capacity of 131.2 mA·h/g at 10C and even 118.6 mA·h/g at 20C. The substitution of W for Ti site can enhance the electronic conductivity of Li4Ti5O12 via the generation of mixing Ti4+/Ti3+, which indicates that Li4Ti4.95W0.05O12 is promising as a high rate anode for the lithium-ion batteries.
Key words: lithium-ion battery; lithium titanate; anode material; doping
1 Introduction
The ever-growing demand for portable batteries with high energy density is exerting pressure for the development of advanced lithium-ion batteries. For large-scale applications such as electric and hybrid vehicle systems, the vital issue is the availability of advanced materials. In recent years, there has been considerable effort devoted to developing high energy density, safe and reliable new materials to use as the anodes in lithium-ion batteries [1]. The spinel Li4Ti5O12 has been found to be an attractive anode material for lithium ion battery [2-3]. In the lithium titanate spinel- type structure of Li4Ti5O12, the formal valence of titanium is +4, which is the highest achievable oxidation state possible for titanium. This Li4Ti5O12 material has been found to intercalate lithium ions without strain or shrinkage to the lattice. It has a flat Li insertion potential at about 1.55 V (versus Li+/Li), above the reduction potential of common electrolyte solvents, mitigating the formation of solid-electrolyte interface (SEI) and avoiding formation of lithium dendrites making the battery safer. However, several disadvantages exist compared to graphite. These include the poor electric conductivity that limits its full capacity at high charge/ discharge rates.
In the literature, effort has been expended to improve the conductivity. Several methods were proposed: 1) doping Li4Ti5O12 by other metal cations or non-metal ions in Li, Ti or O sites [4-9]; 2) incorporating a second phase with high electronic conductivity such as carbon and metal powder [10-15]; 3) making a nitridation to form oxynitride species on its surface [16]. However, to our best knowledge, there are less investigations on the electrochemical characteristics of W-doped Li4Ti5O12 as an anode material. In this work, we proposed to partially substitute Ti4+ with W6+, which will cause a transition of a certain amount of Ti4+ to Ti3+ as charge compensation. The transition from Ti4+ to Ti3+ in Li4Ti5O12 will lead to an increase in the electronic conductivity and thus improve the rate performance.
2 Experimental
2.1 Materials preparation
Li4Ti5O12 samples were prepared using a solid-state method from TiO2 (anatase structure) and LiOH·H2O. The W doped Li4Ti5O12 was also prepared using a solid-state method with TiO2 (anatase structure), LiOH·H2O and WO3. In both cases, 2% (mass fraction) excessive LiOH·H2O was used to compensate for lithium volatilization during the high temperature heating. A 1% W doping level was selected, which yields the composition of Li4Ti4.95W0.05O12 assuming that W sits on a Ti site. This is probably a good assumption that the ionic radius of W6+ was 0.060 nm, similar with the ionic radius of Ti4+ (0.061 nm) and much smaller than the ionic radius of Li+ (0.076 nm). Powders of the precursor materials were mixed in a mortar and pestle with enough methanol to form slurry. The undoped mixed reactant mixture was heated at 800 °C for 5 h in air (oxidizing) and the W-doped mixed reactant mixture was heated at 800 °C for 5 h in 3% (volume fraction) H2/Ar (reducing) to obtain the final powders.
2.2 Characterization
The crystal structure of the powders was characterized by X-ray diffraction (XRD, Rigaka DMax- RB) using Cu Kα radiation (10°≤2θ≤90°). Scanning electron microscope (SEM, KYKY2800) was used to study the morphology of the materials.
2.3 Electrochemical tests
The electrochemical cycling performances of the Li4Ti5O12 powders were evaluated at room temperature (20 °C) with laboratory-scale Li/Li4Ti5O12 button cells including a lithium metal foil as counter electrode, and a composite of 80% (mass fraction) Li4Ti5O12, 10% acetylene black (AB), and 10% polytetrofluornethelene (PTFE) binder as a cathode. A micro-porous polypropylene film (Celgard 2400) was used as a separator and 1 mol/L LiPF6 solution with the 1:1 volumetric ratio of ethylene carbonate-dimethyl carbonate (EC-DMC) was used as the electrolyte. All cells were assembled inside a glove box filled with ultra-pure argon. Charge/discharge characteristics of the cells were recorded in the potential range of 1.0-3.0 V using a LAND batteries test system (CT2001A, Jinnuo Electronics Co., Ltd., Wuhan, China) and specific capacities were calculated based on the mass of Li4Ti5O12. Electrochemical impedance spectroscopy (EIS) was also measured using a potentiostat/galvanostat EG&G 273A coupled to a frequency response analyzer (FRA) EG&G 1025. The impedance data were obtained between 1 MHz and 10 mHz at 5 mV as the applied sinusoidal perturbation. Electrical resistance measurement of the sintered and polished pellets of Li4Ti5O12 was also performed at electrochemical workstation above.
3 Results and discussion
The color of the synthesized Li4Ti5O12 powder is white. The X-ray diffraction patterns of the Li4Ti5O12 and Li4Ti4.95W0.05O12 are shown in Fig. 1(a). All the sharp diffraction peaks can be attributed to the cubic spinel structure of Li4Ti5O12 without obvious impurity phase, which indicates that W6+ has successfully entered the lattice of the spinel and does not change its structural characteristics. The XRD pattern reveals that the (111) peak of the Li4Ti4.95W0.05O12 shifts to smaller angels.For a clear observation, the peak position variation of (111) plane is magnified and shown in Fig. 1(b). The XRD refinement according to the Rietveld method indicates that the lattice parameter of the Li4Ti4.95W0.05O12 is 8.366 ? and the lattice parameter of the Li4Ti5O12 is 8.361 ?. The doping of W will cause the lattice constant of the parameter become larger. This may be due to the fact that the substitution of W6+ for Ti4+ site will cause the transition of a certain amount of Ti4+ to Ti3+ as charge compensation [8], which will cause the increase of the lattice constant of the Li4Ti5O12 because Ti3+ (0.067 nm) is larger than Ti4+ (0.061 nm).
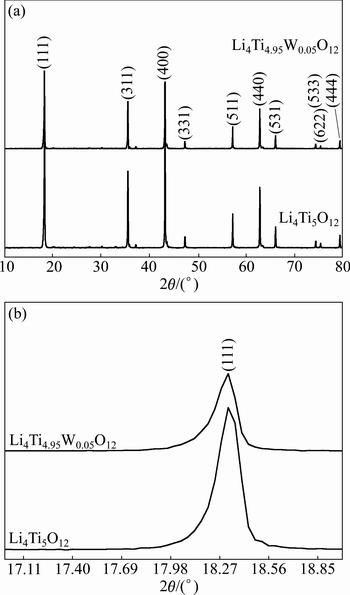
Fig. 1 XRD patterns of Li4Ti5O12 and Li4Ti4.95W0.05O12 (a) and magnified (111) peaks of Li4Ti5O12 and Li4Ti4.95W0.05O12 (b)
Figure 2 shows the SEM images of the undoped Li4Ti5O12 powder and the W-doped powder. The grains of pure Li4Ti5O12 are small with the size generally distributed in the range of 200-300 nm. Small particles will enlarge the contact areas between particles and electrolyte, and thus improve the specific capacity of the electrode. In Fig. 2(b), the particle size of the W-doped powder is larger than that of the pure one, but is also several hundred nanometers.
The acquisition of these homogeneous, nano-sized Li4Ti5O12 powders could be attributed to the precursor material, nano-sized anatase structure TiO2, which could be mixed more sufficiently with the other materials compared to the usually used micro-sized rutile structure TiO2. The sintering time in this work is much shorter than the ordinarily reported 10-15 h. Short sintering time is also critical to prevent the grains from excessive growth.
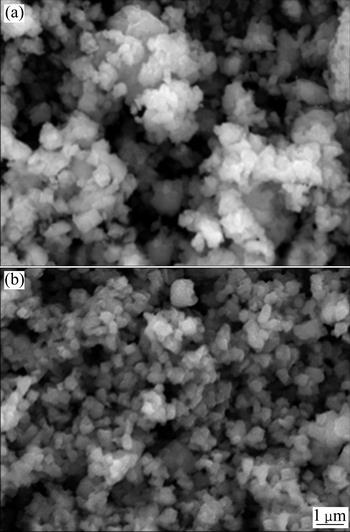
Fig. 2 SEM images of Li4Ti5O12 (a) and Li4Ti4.95W0.05O12 (b)
The electrochemical properties of the powders are determined by charge/discharge test at constant current density. Figure 3 shows the first and second charge-discharge curves of the undoped and W-doped Li4Ti5O12 powder at 0.5C rate in potential window between 3.0 and 1.0 V. The cycling behavior is typical of LTO with a flat plateau at an average potential of 1.55 V which is attributed to a two-phase phenomenon pertaining to Li4Ti5O12 and Li7Ti5O12 phases. The voltage platform is the result of an adjustment of the above intercalation and deintercalation processes. The sharp and linear increases of the voltage at the end of the charge and the start of the discharge curves are results of electrode polarization. The cation distribution in Li4Ti5O12 and Li7Ti5O12 phases during electrochemical charge/discharge processes can be written as follows:
[Li]8a[Li1/3Ti5/3]16d[O4]32e+Li++e-=
[Li2]16c[Li1/3Ti5/3]16d[O4]32e (1)
During the first discharge, undoped Li4Ti5O12 takes about 2.79 Li atoms corresponding to a capacity of 162.4 mA·h/g that matches well with the expected theoretical capacity for Li4Ti5O12. However, the W-doped Li4Ti5O12 takes about 2.66 Li atoms corresponding to a capacity of 154.5 mA·h/g less than that of the undoped one. A small electrode polarization of about 0.02 V is observed between charge-discharge curves, indicating the existence of good interparticle electrical contacts and better ion transport. In Li4Ti5O12 structure, 75% Li+ ions locate at tetrahedral 8a sites, 25% Li+ and Ti4+ are randomly distributed at octahedral 16d sites, O2- ions occupy the 32e sites, and the 8b, 48f and 16c sites are empty [2]. During lithiation process, 3 Li-ions can be accommodated by Li4Ti5O12, which will make spinel Li4Ti5O12 transform to rock-salt Li7Ti5O12. In this process, all Li-ions, including that in 8a site and the newly inserted, will move and occupy the 16c site, and then all octahedral sites are filled. W-doping may impede the transportation of Li-ions from 8a to 16c sites, and thus influence the insertion of outside Li-ions into Li4Ti5O12 structure. As a result, the capacity of W-doped Li4Ti5O12 is decreased.
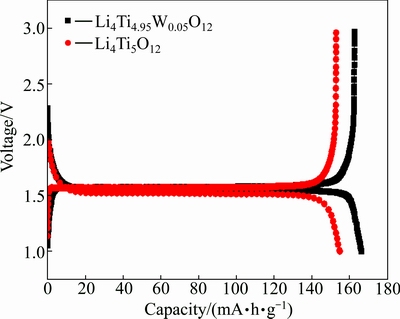
Fig. 3 Initial discharge-charge curves of Li4Ti5O12 and Li4Ti4.95W0.05O12 at 0.5C rate
High rate performance is one of the most important electrochemical characteristics of lithium ion batteries for HEV application. The charge/discharge capacity of pure Li4Ti5O12 and W-doped Li4Ti5O12 electrodes at different current rates from 0.5C to 20C are shown in Fig. 4. It can be seen that, the rate performance of Li4Ti5O12 can be improved by the doping of W. The charge and discharge capacities decrease with the current rate increasing. The Li4Ti4.95W0.05O12 exhibits an excellent rate capability compared with the Li4Ti5O12, although Li4Ti5O12 shows much more initial capacity than Li4Ti4.95W0.05O12. At 0.5C, the Li4Ti4.95W0.05O12 presents a discharge capacity of 154.5 mA·h/g, while the Li4Ti5O12 exhibits a discharge capacity of 162.4 mA·h/g. At 20C, the discharge capacity of the Li4Ti4.95W0.05O12 still remains 118.6 mA·h/g, while the capacity of the undoped Li4Ti5O12 is only 69.2 mA·h/g. We propose to partially substitute Ti4+ with W6+, which will cause a transition of a certain amount of Ti4+ to Ti3+ as charge compensation. The transition from Ti4+ to Ti3+ in Li4Ti5O12 will lead to an increase in the electronic conductivity and thus improve the rate performance.
The cycling performances of the W-doped and undoped Li4Ti5O12 samples at the rate of 1C are exhibited in Fig. 5. As is seen Fig. 5, more than 10.7% of the capacity loss occurs in the undoped Li4Ti5O12 at 1C after 100 cycles, while the W-doped Li4Ti5O12 loses only 2.5% after 100 cycles. The 100th discharge capacity of the W-doped Li4Ti5O12 electrode is 150.4 mA·h/g, but the corresponding value of the Li4Ti5O12 electrode is decreased to 144.6 mA·h/g. This indicates that the introduction of W ions can increase the cycle stability. This suggests that the electrode polarization is probably decreased by W-doping.
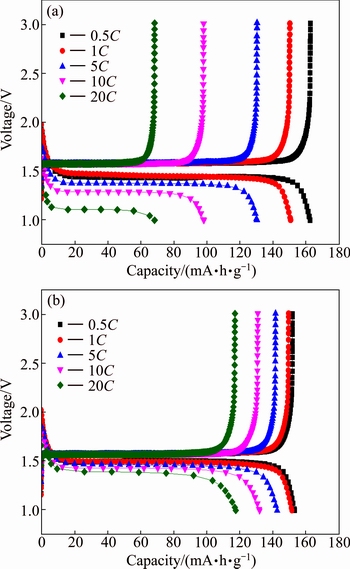
Fig. 4 Initial discharge-charge curves of Li4Ti5O12 (a) and Li4Ti4.95W0.05O12 (b) at different rates (0.5C, 1C, 5C, 10C, 20C)
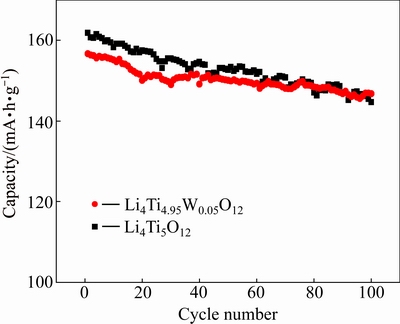
Fig. 5 Cycling performance of Li4Ti5O12 and Li4Ti4.95W0.05O12 samples (current rate, 1C)
Electrochemical impedance spectroscopy (EIS) may be considered as one of the most sensitive tools for studying the changes in the electrode behavior due to surface modification. EIS results of the coin cells with the Li4Ti5O12 and W-doped Li4Ti5O12 cathodes are shown in Fig. 6. The measurement is carried out after the cells have been discharged to the depth of 50% followed by three cycles. The impedance spectra are composed of one semicircle at higher frequencies followed by linear part at lower frequency end. The semicircle in the high region represents the migration of the lithium ions at the electrode/electrolyte interface. The low frequency region of the straight line is attributed to the diffusion of the lithium ions into the bulk of the electrode material, the so-called Warburg diffusion [8].
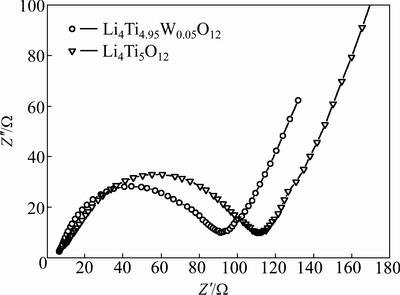
Fig. 6 EIS of Li4Ti5O12 and Li4Ti4.95W0.05O12 samples
The relationship between the imaginary impedance and the low frequencies is governed by Eq. (2). The diffusion coefficient values of the lithium ions in the bulk electrode materials are calculated by Eq. (3). The Warburg impedance coefficient can be obtained from the straight lines [7]. The relation is governed by Eq. (4). These calculated results are recorded in Table 1.
Z″=-σwω-1/2 (2)
D=0.52 (3)
Zre=Re+Rct+σwω-1/2 (4)
where Z″ is the imaginary impedance, ω is the angular frequency in the low frequency region, Rct is the charge-transfer resistance, Re is the electrolyte resistance, D is the diffusion coefficient.
The obtained diffusion coefficients show that W-doped Li4Ti5O12 sample has higher mobility for Li+ diffusion than the undoped Li4Ti5O12 sample. This is attributed to the concentration of electrons caused by the transition of a certain amount of Ti4+ to Ti3+ as charge compensation. The transition from Ti4+ to Ti3+ in Li4Ti5O12 will lead to an increase in the electronic conductivity and thus improve the rate performance.
Table 1 Impedance parameters of undoped Li4Ti5O12 and Li4Ti4.95W0.05O12 samples

4 Conclusions
1) Li4Ti4.95W0.05O12 powders have been successfully synthesized by a simple solid state reaction. XRD patterns show that the Li4Ti4.95W0.05O12 has good crystallinity and high phase purity. The Li4Ti4.95W0.05O12 electrode presents better cycling performance than the Li4Ti5O12 electrode prepared by the similar process. W-doping does not change the phase composition and particle morphology, while remarkably improves its cycling stability at high charge/discharge rate.
2) The substitution of W for Ti site can enhance the electronic conductivity of Li4Ti5O12 via the generation of mixing Ti4+/Ti3+. At 20C, the discharge capacity of the Li4Ti4.95W0.05O12 still remains 118.6 mA·h/g, while the capacity of the undoped Li4Ti5O12 is only 69.2 mA·h/g. More than 10.7% of the capacity loss occurs in the undoped Li4Ti5O12 at 1C after 100 cycles, while the W-doped Li4Ti5O12 loses only 2.5% after 100 cycles. The 100th discharge capacity of the W-doped Li4Ti5O12 electrode is 150.4 mA·h/g, but the corresponding value of the Li4Ti5O12 electrode is decreased to 144.6 mA·h/g.
References
[1] TARASCON J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries [J].Nature, 2001, 414(15): 359-367.
[2] OHZUKU T, UEDA A, YAMAMOTO N. Zero-strain insertion material of Li[Li1/3Ti5/3Ti5/3]O4 for rechargeable lithium cells [J]. Journal of the Electrochemical Society, 1995, 142(5): 1431-1435.
[3] ARIYOSHI K, YAMATO R, OHZUKU T.Zero-strain insertion mechanism of Li[Li1/3Ti5/3]O4 for advanced lithium-ion (shuttlecock) batteries [J]. Electrochim Acta, 2005, 51(6): 1125-1129.
[4] MARTLN P, LOPEZ M L, PICO C, VEIGA M L. Li(4-x)/3Ti(5-2x)/3CrxO4 (0≤x≤0.9) spinels: New negatives for lithium batteries [J]. Solid State Sci, 2007, 9(6): 521-526.
[5] HAO Y J, LAI Q Y, LU J Z, JI X Y. Effects of dopant on the electrochemical properties of Li4Ti5O12 anode materials [J]. Ionics, 2007, 13(5): 369-373.
[6] HUANG S, WEN Z, ZHU X, LIN Z. Effects of dopant on the electrochemical performance of Li4Ti5O12 as electrode material for lithium ion batteries [J]. J Power Sources, 2007, 165(1): 408-412.
[7] LI X, QU M, YU Z. Structural and electrochemical performances of Li4Ti5-xZrxO12 as anode material for lithium-ion batteries [J]. J Alloys Compd, 2009, 487(1/2): L12-L17.
[8] ZHONG Z. Synthesis of Mo4+ substituted spinel Li4Ti5-xMoxO12 [J]. Electrochem Solid-State Lett, 2007, 10(12): A267-A269.
[9] ALLEN J L, JOW T R, WOLFENSTINE J. Low temperature performance of nanophase Li4Ti5O12 [J]. J Power Sources, 2006, 159(2): 1340-1345.
[10] HUANG S, WEN Z, ZHANG J, GU Z, XU X. Preparation and electrochemical performance of Ag doped Li4Ti5O12 [J]. Solid State Ionics, 2006, 177(15): 851-855.
[11] GAO J, YING J, JIANG C, WAN C. Preparation and characteristic of carbon-coated Li4Ti5O12 anode material [J]. J Power Sources, 2007, 166(2): 255-259.
[12] YANG L, GAO L. High-density spherical Li4Ti5O12/C anode material with good rate capability for lithium ion batteries [J]. J Alloys Compd, 2009, 485(3): 93-97.
[13] CHENG L, YAN J, ZHU G N, LUO J Y, WANG C X, XIA Y Y. General synthesis of carbon-coated nanostructure Li4Ti5O12 as a high rate electrode material for Li-ion intercalation [J]. J Mater Chem, 2010, 20(3): 595-602.
[14] WANG Y, LIU H, WANG K, EIJI H, WANG Y, ZHOU H. Synthesis and electrochemical performance of nano-sized Li4Ti5O12 with double surface modification of Ti(III) and carbon [J]. J Mater Chem, 2009, 19(37): 6789-6785.
[15] KAVAN L, DUNSCH L, KATAURA H. Electrochemical tuning of electronic structure of carbon nanotubes and fullerene peapods [J]. Carbon, 2004, 42(5): 1011-1019.
[16] PARK K S, BENAYAR A, KANG D J, DOO S G. Nitridation-driven conductive Li4Ti5O12for lithium ion batteries [J]. J Am Chem Soc, 2008, 130(45): 14930-14931.
(Edited by HE Yun-bin)
Received date: 2012-10-26; Accepted date: 2013-03-08