Ferrocenyl building block constructing porous organic polymer for gas capture and methyl violet adsorption
来源期刊:中南大学学报(英文版)2020年第4期
论文作者:刘清泉 黄晶 谭志强 苏惠敏 郭翼雯 刘欢 廖博
文章页码:1247 - 1261
Key words:ferrocene; porous organic polymer; gas capture; dyeing wastewater
Abstract: Ferrocene-based porous organic polymer (FcPOP) was constructed with ferrocene and porphyrin derivatives as building blocks via Schiff-base coupling. FcPOP was well characterized, and exhibited good thermal stability, high porosity, microporous structure, and homogeneous pore size distribution. Ferrocene blocks with highly electron-rich characteristics endowed FcPOP with excellent adsorption capacity of CO2 and methyl violet. The kinetic study indicated adsorption of methyl violet onto FcPOP mainly complied with pesudo-second order model. The maximum adsorption capacity of FcPOP derived from Langmuir isotherm model reached up to 516 mg/g. More importantly, FcPOP could be easily regenerated and repeatedly employed for removal of methyl violet with high efficiency. Overall, FcPOP in the present study highlighted prospective applications in the field of gas capture and dyeing wastewater treatment.
Cite this article as: HUANG Jin, TAN Zhi-qiang, SU Hui-min, GUO Yi-wen, LIU Huan, LIAO Bo, LIU Qing-quan. Ferrocenyl building block constructing porous organic polymer for gas capture and methyl violet adsorption [J]. Journal of Central South University, 2020, 27(4): 1247-1261. DOI: https://doi.org/10.1007/s11771-020-4364-4.

J. Cent. South Univ. (2020) 27: 1247-1261
DOI: https://doi.org/10.1007/s11771-020-4364-4
HUANG Jin(黄晶)1, 3, TAN Zhi-qiang(谭志强)1, 2, SU Hui-min(苏惠敏)1, 2, GUO Yi-wen(郭翼雯)1, 2,
LIU Huan(刘欢)1, 2, LIAO Bo(廖博)1, 2, LIU Qing-quan(刘清泉)1, 2, 3
1. Schoolof Materials Science and Engineering, Hunan University of Science and Technology,Xiangtan 411201, China;
2. Hunan Provincial Key Lab of Advanced Materials for New Energy Storage and Conversion,Xiangtan 411201, China;
3. Hunan Provincial Key Lab of Controllable Preparation and Functional Application of Fine Polymers, Xiangtan 411201, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract: Ferrocene-based porous organic polymer (FcPOP) was constructed with ferrocene and porphyrin derivatives as building blocks via Schiff-base coupling. FcPOP was well characterized, and exhibited good thermal stability, high porosity, microporous structure, and homogeneous pore size distribution. Ferrocene blocks with highly electron-rich characteristics endowed FcPOP with excellent adsorption capacity of CO2 and methyl violet. The kinetic study indicated adsorption of methyl violet onto FcPOP mainly complied with pesudo-second order model. The maximum adsorption capacity of FcPOP derived from Langmuir isotherm model reached up to 516 mg/g. More importantly, FcPOP could be easily regenerated and repeatedly employed for removal of methyl violet with high efficiency. Overall, FcPOP in the present study highlighted prospective applications in the field of gas capture and dyeing wastewater treatment.
Key words: ferrocene; porous organic polymer; gas capture; dyeing wastewater
Cite this article as: HUANG Jin, TAN Zhi-qiang, SU Hui-min, GUO Yi-wen, LIU Huan, LIAO Bo, LIU Qing-quan. Ferrocenyl building block constructing porous organic polymer for gas capture and methyl violet adsorption [J]. Journal of Central South University, 2020, 27(4): 1247-1261. DOI: https://doi.org/10.1007/s11771-020-4364-4.
1 Introduction
The design, synthesis and application of porous polymers have generated enormous interest and been research hot spots during the past decades due to their wide application in the field like gas capture [1], water purification [2], metal ion enrichment [3], separation [4], and heterogeneous catalysis [5]. Several kinds of porous polymers have been developed such as covalent organic frameworks (COFs) [6], polymer with intrinsic microporosity (PIMs) [7], hypercrosslinked polymers (HCPs) [8], and porous organic polymers (POPs) [5, 9], porous coordination polymers (PCPs) [10, 11]. Typically, structure diversity of building monomer and extensively active groups dramatically improved design flexibility of pore channel and skeleton of POPs, which made it one of the most important platforms to developing novel porous polymers [12]. In comparison with PCPs, POPs showed excellent chemical stability under harsh environment. Research progress [13-15] in recent years suggests that taking full advantage of nanoporous channels of POPs can possess great potential in developing novel functional materials to solve some challenging issues in the field of energy and environment.
Metal-containing POPs often displayed unique properties due to introducing metal elements into polymer networks. For example, theoretical and experimental studies elucidated that doping the host-material surface with metals such as Li, Na, K, Ca was a promising choice to enhance gas uptake of host materials [16, 17]. Furthermore, introducing transition metal ions opened the possibility to generate nanoporous materials with additional electrical, optical, catalytic active center, or magnetic properties. There are two synthetic routes to produce metal-containing POPs. One is post-metallization of POPs via introducing carboxyl group or sulfonic group into the networks firstly, and then metallizing the networks via metal ion exchange [17]. Post-metallization of POPs usually resulted in partial loss of surface area. The other is the preparation of POPs via metal-containing building block like metalloporphyrin derivative [18]. Various organometallics with at least two active groups can be used as a building block, and greatly facilitate the generation of metal-containing POPs.
Ferrocene and its derivatives are a kind of typical organometallic compounds, and have sandwich structure with highly electron-rich and excellent thermal stability [19]. Ferrocene derivatives have been used as a rigid building block to construct POPs, which displayed excellent gas storage capacity [20-22]. In fact, highly electron- rich ferrocene is expected to have strongly interaction with cationic organic compound such caitonic dyes, and can be applied in the field of dyeing wastewater purification. With the background in mind, we prepared ferrocenyl POP (FcPOP) with 1,1'-ferrocenedicarboxaldehyde and 5,10,15,20-tetrakis(4-aminophenyl)-21H,23H- porphine as rigid building blocks via Schiff-base reaction, and attempted to systematically investigate adsorption behavior of methyl violet onto FcPOP.
2 Experiments
2.1 Materials
4-nitrobenzaldehyde(97%), pyrrole(99%) and SnCl2·2H2O(98%) were purchased from Aladdin (China). Dimethylsulfoxide (DMSO), methanol, acetone, tetrahydrofuran(THF), aceticanhydride, propionic acid, pyridine, dichloromethane, and methyl violet were obtained from Sinopharm Chemical Reagent Co. Ltd. (China). Anhydrous DMSO was refluxed by CaH2 and distilled in reduced pressure. 5,10,15,20-tetrakis(4-aminophenyl)- 21H,23H-porphine was synthesized by the method described in the literature with slight modifications [18]. 1,1’-Ferrocenedicarboxaldehyde was also provided by Aladdin (China).
2.2 Synthesis of 5,10,15,20-tetrakis(4- aminophenyl)-21H,23H-porphine
4-nitrobenzaldehyde (22.0 g, 0.145 mol) and aceticanhydride (24.0 mL, 0.254 mol) were dissolved in propionic acid (600 mL). The solution was then refluxed, to which pyrrole (10.0 mL, 0.144 mol) was slowly added. After refluxing for 30 min, the mixture was cooled to give a precipitate which was collected by filtration, washed with H2O and methanol, and dried under vacuum. The resulting powder was dissolved in pyridine (160 mL), and then was heated to reflux for 1 h. After being cooled to room temperature, the precipitate was collected by filtration and washed with acetone to give 5,10,15,20-tetrakis(4- nitrophenyl)-21H, 23H-porphine as a purple crystal in 14% yield. The product (4.13 g, 5.19×10-3 mol) was dissolved in hot dilute HCl (500 mL) at 70 °C, to which SnCl2·2H2O (18.0 g, 7.97×10-2 mol) was added. The resulting mixture was stirred at 70 °C for 30 min and then cooled to 0 °C. After neutralized with aqueous ammonium, the gray precipitate was collected as the crystalline product by filtration. The product was dissolved in THF, and the purple crystal of 5,10,15,20-tetrakis(4- aminophenyl)-21H,23H-porphine was obtained by rotary evaporation and vacuum drying. 1H NMR (300 MHz, CDCl3) δ (ppm): 2.7 (s, 2H), 4.0 (s, 8H), 7.1–8.0 (m, 16H), 8.9 (s, 8H)
2.3 Synthesis of FcPOP
In a 100 mL three-neck flask with magnetic stirring bar, 5,10,15,20-tetrakis(4-aminophenyl)- 21H,23H-porphine (276.8 mg, 0.41 mmol) and 1,1'-ferrocenedicarboxaldehyde (198.5 mg,0.82 mmol) were suspended in anhydrous dimethyl sulfoxide (40 mL). The flask was then degassed by freeze-pump-thaw technique three times in a liquid nitrogen bath. Finally, the flask was evacuated to an internal pressure of 150 mtorr. The reaction was carried out at 180 °C for 72 h to obtain a black solid, which was filtered and washed with methanol, acetone, and THF, respectively. The black solid was further extracted by Soxhlet extractor with THF for 24h, and methanol for 24h, respectively. The final product was dried in a vacuum oven for 12 h at 100 °C to afford black powder (64.8 mg, 64.8% yield based on 1,1'-ferrocenedicarboxaldehyde).
2.4 Adsorption of methyl violet (MV)
The adsorption property of FcPOP was evaluated by adsorption experiments using methyl violet (MV), which was dissolved in water to obtain 65.8 mg/L solution. All adsorption experiments were stirred at the speed of 300 r/min and 298 K.
Adsorption kinetics can reveal the relation between adsorbent structure and its adsorption property, and the adsorption process and result can be analyzed and predicted according to the kinetic model. The adsorption kinetic experiments were carried out with 5.0 mL MV solution and 10 mg FcPOP, and MV concentration was detected with a UV spectrophotometer at an interval of 5 min. The pseudo-first order and pseudo-second order models rearranged by ZHAN et al [23] are employed to investigate adsorption kinetics of MV onto FcPOP, and the models are provided as follows:
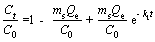 (1)
(1)
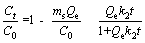 (2)
(2)
Eqs. (1) and (2) are pseudo-first order and pseudo-second order models of adsorption kinetics, respectively, where C0 and Ct (mg/L) are the initial MV concentration and the concentration at time t; ms (g/L) is the concentration of adsorbent; k1 and k2 are the rate constants of pseudo-first order and pseudo-second order models, respectively; and Qe is the equilibrium adsorption capacity.
The equilibrium adsorption isotherm was implemented with 5 mg FcPOP and 8 mL MV solution with the initial concentration varied from 65.8 to 394.8 mg/L. The mixtures were stirred for 6 h to assure that the adsorption reached equilibrium. Equilibrium adsorption capacity and equilibrium MV concentration were calculated from the initial and final MV concentrations. Langmuir [24], Freundlich [25], and Redlich-Peterson [26] models were employed to fit the experimental data of adsorption isotherm.
Langmuir isotherm model:
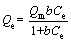 (3)
(3)
where Qm (mg/g) is the maximum adsorption capacity based on monolayer adsorption; b is the Langmuir constant; Ce (mg/L) is the equilibrium MV concentration.
Freundlich isotherm model.
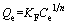 (4)
(4)
where KF and n are the Freundlich constants in relationship with the sorption capacity and sorption intensity, respectively.
Redlich-Peterson isotherm model:
 (5)
(5)
where KR and α are the Redlich-Peterson constants, and β is the coefficient from 0 to 1.
The cyclic adsorption/desorption experiments were conducted with 5.0 mL MV solution (65.8 mg/L) and 10 mg FcPOP. The mixtures were stirred at the speed of 300 r/min for 6 h, and then centrifugal separation was employed to remove MV solution. FcPOP were regenerated by methonal/ water co-solvent with NaCl [27].
2.5 Characterization
FT-IR spectrum was collected in transmission mode in KBr pellets at room temperature on a FTIR-2000 spectrometer (Perkin-Elmer in America). 1H-NMR Characterization was performed on a Varian 400-MR spectrometer, operating at 400 MHz. 13C cross-polarization, magic angle spinning (CP/MAS) nuclear magnetic resonance spectra were recorded on a AVANCE III 400 MHz spectrometer (BRUKER Inc., Switzerland) equipped with a 4-mm HXY T3 PENCIL probe.
The thermogravimetric analyses (TGA) were carried out under N2 flow on a Mettler TGA/ SDTA851 thermogravimetric analysis instrument. Sample was heated at a rate of 5 °C/min from 35 to 1000 °C under a nitrogen atmosphere.
Microstructures of FcPOP were examined by high resolution transmission electron microscopy (HR-TEM, JEOL JEM-2000EX). Surface morphology was observed by scanning electron microscopy (SEM, JSM-6380 LV).
The X-ray photoelectron spectroscopy (XPS) experiment was performed on a K-Alpha 1063 spectrometer (Thermo Fisher Scientific) and the core level spectrum was measured using a monochromatic Al Kα X-ray source (hv=1386.6 eV).
Nitrogen sorption porosimetry was performed on a micromeritics ASAP 2020 volumetric adsorption analyzer. The experiments were carried out at the temperature of liquid nitrogen (77.3 K). The samples were first heated in a tube under vacuum at 120 °C for 20 h to remove adsorbed materials. Pore size distribution curve and the pore volume were derived from the adsorption branches of the isotherms using the nonlocal density functional theory (NLDFT). CO2 and CH4 adsorption experiments were conducted at 273 K and 298 K, respectively.
MV concentration was determined by UV751GW ultraviolet spectrophotometer based on a standard linear curve, which was constructed by the relationship between UV adsorption intensity at 569 nm and a series of MV solution with known different concentration.
3 Results and discussion
3.1 Synthesis and characterization of FcPOP
Synthesis route 1HNMR spectrum of 5,10,15,20-tetrakis(4-aminophenyl)-21H, 23H-porphine was introduced in supporting information (Scheme S1 and Figure S1). Ferrocene- based porous organic polymer (FcPOP) was synthesized by Schiff-base coupling of 1,1'-ferrocenedicarboxaldehyde and 5,10,15,20-tetrakis (4-aminophenyl)-21H,23H- porphine, and the synthetic route is shown in Scheme 1.
The chemical structure of FcPOP was investigated by FTIR (Figure S2) and 13C CP/MAS NMR (Figure 1). FT-IR spectrum of FcPOP exhibited a characteristic stretching vibration of C=N at 1600 cm-1, which was characteristic of vC=N moieties. The absorption at 2970 cm-1 and 1410 cm-1 was assigned to C—H stretching of ferrocene and the cyclopentadienyl(Cp) rings bending, respectively. In addition, the stretching vibration of C=O in 1,1’-ferrocene- dicarboxaldehyde at 1680 cm-1 disappeared in the spectrum of FcPOP. The solid-state 13C CP/MS NMR spectrum displayed multiple broad peaks with different strength. In particular, the signals at 61.12ppm, 65.09ppm, 68.53ppm, 69.97ppm and 76.12ppm were ascribed to five kinds of carbon in cyclopentadienyl(Cp) rings; and signals at 118.906ppm, 141.82ppm and 152.73ppm were derived from the carbons of porphyrin rings. The characteristic peaks of carbons in C=N appeared at 156.45ppm.

Scheme 1 Synthetic route for ferrocene-based porous organic polymer (FcPOP)

Figure 1 Solid-state 13C CP/MAS NMR spectrum of FcPOP
XPS analysis can provide more information about the elemental composition of FcPOP surfaces (Figure 2). The transitions energy of around 707.4 eV and 720 eV corresponded in shape and position to Fe 2p 3/2 and Fe 2p 1/2 bands in ferrocenyl blocks, respectively. XPS result of FcPOP was in accordance with the previous XPS study of ferrocene [28]. The C/Fe molar ratio from XPS spectrum was calculated to be 47/1, which was higher than the theoretical value of 34/1 derived from the feed ratio of raw materials. We speculated that XPS method only analyzed the elemental composition of FcPOP surface, which was different from that of FcPOP bulk.
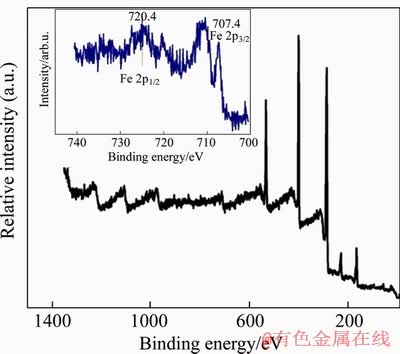
Figure 2 X-ray photoelectron spectroscopy (XPS) graph of FcPOP
Overall, the results of FT-IR, 13C CP/MS NMR and XPS analysis demonstrated that ferrocenyl units were successfully incorporated into polymer networks as a building block.
The morphology of FcPOP was investigated by scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HR-TEM), as shown in Figure 3. SEM image revealed that FcPOP was random granules with particle size of 1-3 μm. HR TEM image displayed FcPOP was amorphous, which was further demonstrated by XRD curves (Figure S3). Interesting, a number of ultra-micropores could be observed in TEM image (the red circles in Figure 3(b)). Furthermore, elemental distribution maps of carbon, nitrogen, and iron were also obtained by energy dispersive spectrometer (Figures 3(c)-(e)). Expectedly, carbon, nitrogen, and iron elements were uniformly distributed in FcPOP.
As-prepared FcPOP was found to be insoluble in any organic solvent, such as dimethyl sulphoxide, dimethylformamide, and tetrahydrofuran. Moreover, there was no mass loss or BET surface area decrease after FcPOP was soaked in HCl or NaOH solution (~10 wt%) for at least 12 h. The facts suggested that FcPOP has an excellent physicochemical stability. The thermal stability was investigated by thermogravimetric analysis under nitrogen atmosphere (Figure S4). The results indicated that FcPOP remained stable up to approximately 230 °C, and the mass loss below 230 °C was believed to be water or/and residual solvent loss. The thermal stability of FcPOP was similar to previous porous organic polymers, such as CMPs [4], HCPs [29], COFs [6], and PIMs [30].

Figure 3 SEM (a), TEM(b), and elemental images (c, d, e) (from 100 μm×100 μm region) of FcPOP
3.2 Pore structure and gas capture of FcPOP
To demonstrate porous architecture of FcPOP effectively constructed with ferrocene and porphyrin derivatives as building blocks, nitrogen adsorption/desorption measurement was carried out to evaluate the permanent porosity of FcPOP, and the isotherm is provided in Figure 4(a). Obviously, the isotherm exhibited type I profile according to IUPAC classifications [31]. The N2 uptake at low pressure (p/p0=0-0.01) experienced a sharp increase, suggesting a large number of permanent micropores in FcPOP. The surface area of FcPOP was calculated to be 602 m2/g (Figure S5), which was comparable to similar POPs like PECONF-2 (637 m2/g) [32] and PTPA-3 (530 m2/g) [33], but lower than that of COFs, such as COF-5 (1590 m2/g) [34] and COF-10 (1760 m2/g) [35]. In addition, the pore volume of FcPOP was calculated to be 0.4 cm3/g at relative pressure of p/p0=0.99, and the micropore volume was 0.12 cm3/g, which was derived from t-plot method. Pore size distribution curve of FcPOP (Figure 4(b)) was calculated using nonlocal density functional theory (NLDFT). The network exhibited a narrow pore size distribution and a large number of pores in the micropore region extending to the lower part of the mesopore region. The average pore diameter of 1.6 nm could also demonstrate the predominant microporous nature in FcPOP.
The excellent porosity of metal-doping FcPOP promoted us to estimate their gas storage property. Determination of CO2, CH4 and N2 uptake was carried out at 273 K/1.0 bar and 298 K/1.0 bar, respectively, and the isotherms are shown in Figure 4(c). The volumetric CO2 uptake of FcPOP was 2.54 mmol/g (11.2 wt%) at 273 K and 1.63 mmol/g (7.2 wt%) at 298 K. The value at 273 K was higher than PPAF-34 (1.39 mmol/g, 953 m2/g) [36] and CMP-SO-1B2 (2.13 mmol/g, 1085 m2/g) [37] under the same conditions although the surface area of FcPOP was lower than that of PPAF-34 or CMP-SO-1B2. The excellent CO2 capacity of FcPOP may be attributed to highly electron-rich ferrocene blocks, which could enhance affinity of FcPOP for electron-poor adsorbate of CO2.
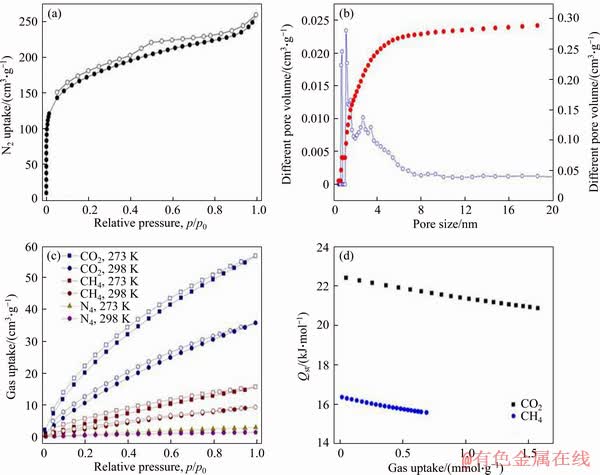
Figure 4 Nitrogen adsorption (filled symbols) and desorption (empty symbols) isotherm measured at 77 K (a); Pore size distribution curve for FcPOP calculated by NL-DFT (b); Gas uptake performance of FcPOP (c); CO2 and CH4 isosteric heats of FcPOP (d)
FcPOP exhibited moderate CH4 capture capacity of 0.7 mmol/g at 273 K and 0.42 mmol/g at 298 K, respectively. To thoroughly investigate the host-guest interaction, Qst (isosteric heat of adsorption) value of CO2 and CH4 were calculated using the virial model [38]. The virial equation fitting curves of CO2 and CH4 are provided in Figures S6 and S7, respectively. The CO2 and CH4 isosteric heats of FcPOP are shown in Figure 4(d). At low gas loading, the Qst value mainly reflected the interaction strength between adsorbate and adsorbent, and was found to be 22.4 and 16.3 kJ/ mol for CO2 and CH4, respectively. The Qst value of CO2 was the same level to many other porous polymers, such as porous electron-rich covalent organic frameworks [32], PTPA-3 [33], and PAFs [37]. Interestingly, Qst only decreased slightly with increasing CO2 loading of FcPOP. This fact indicated that comparatively strong interaction of CO2 with FcPOP are kept during adsorption of CO2 by adsorbate. The Qst value of CH4 was lower than that of CO2, indicating weaker host-guest interaction.
To assess the potential application of FcPOP in the field of gas separation, selectivities of CO2 over CH4 and N2 were investigated, respectively. The ideal adsorption of solution theory (IAST) model was applied to calculating CO2/CH4 and CO2/N2 ideal selectivities based on the initial slopes of adsorption isotherms derived from 273 K and 298 K, and the results are shown in Figure S8 and Table S1. FcPOP exhibited moderate selectivities for CO2/CH4 (5.39/273 K, 4.38/298 K), and excellent selectivities for CO2/N2 (29.81/273 K, 24.85/298 K), which was comparable to the results reported by COOPER et al [39] and JIANG et al [40]. The highly elcetron-rich ferrocenyl blocks in FcPOP may be favorable to improve the interaction between polarizable CO2 molecules via hydrogen bonding and/or dipole-quadrupole interactions. The fact can be responsible for the excellent CO2 ideal selectivity.
3.3 Removal of methyl violet from aqueous solution
The as-prepared FcPOP have excellent physicochemical stability, a narrow pore size distribution, and electron-rich conjugated structure with ferrocene and porphyrin units, which is favorable to improve the interaction with small organic molecules, and can find application in removal of organic molecules from wastewater. Consequently, in the present manuscript, methyl violet (MV) is used as a target dye to investigate the adsorption capacity and adsorption rate of FcPOP. MV is a typical cationic dye, and widely used as coloring agents for textile and leather materials. The experimental data, the adsorption kinetic curves derived from pesudo-first order and pesudo-second order model [23] are shown in Figure 5, and the parameters calculated from model fitting are provided in Table 1.
In the experiment of adsorption kinetics, FcPOP (10 mg) was employed to treat 5.0 mL MV aqueous solution with initial concentration of 65.8 mg/L. MV concentration of aqueous solution was monitored by UV absorption intensity at 569 nm according to the equation from the fitted standard curve (Figure S9), and UV curves recorded at an interval of 5 min were provided in Figure S10. MV was rapidly adsorbed by FcPOP, and the adsorption equilibrium reached within 30 min. The removal efficiency of MV was about 98.9%, and the purple solution gradually turned to colourless.

Figure 5 Adsorption experimental data, and kinetic curves derived from pesudo-first and pesudo-second order models
Table 1 Pesudo-first and pesudo-second order parameters and correlation coefficient

The equilibrium adsorption capacity Qe values derived from pesudo-first and pesudo-second order models were 32.58 and 37.18 mg/g, respectively. The values were close to the experimental data, which are calculated to be 32.72 mg/g from Figure 5. The fact illustrated that Qe could be predicted by the kinetic models. Similar to chemical reaction order, there are two factors like dye concentration and adsorbent property having effect on the adsorption rate of pesudo-second order model. However, only one fact such as dye concentration or adsorbent property, has impact on the adsorption rate of pesudo-first order model. Moreover, a plateau could be observed in the fitting curve of pesudo-first order model with decreasing MV concentration, but there was still a reduced trend with increasing adsorption time in the curve from pesudo-second order model. The correlation coefficient from pesudo-second order model was slightly higher than that of the former, indicating that MV adsorbed by FcPOP mainly complied with pesudo-second order model. The interaction between electron-rich conjugated structure in FcPOP with cationic MV molecules prompted that both MV concentration and FcPOP adsorption property had important effect on the adsorption rate.
Experimental data of equilibrium adsorption isotherm are shown in Figure 6. The equilibrium adsorption capacity of FcPOP dramatically improved with increasing MV concentration. However, the rising trend diminished, and gradually, the adsorption capacity of FcPOP became saturated. Non-linear models of Langmuir, Freundlich, and Redlich-Peterson [24-26] were employed to fit the experimental data of MV adsorbed onto FcPOP. The fitting curves are also provided in Figure 6. Parameters and the correlation coefficient of various models are provided in Table 2.
The fitting results displayed that the correlation coefficient of Redlich-Peterson model was higher than that of Langmuir or Freundlich models. The fact suggested that the adsorption process of MV onto FcPOP was more accordant with the adsorption isotherm of Redlich-Peterson model, which is in noncompliance with the ideal monolayer adsorption of Langmuir model, and more applicable to physical and chemical adsorption of inhomogeneous surface. Consequently, it can be concluded that the main adsorption site of FcPOP was the inhomogeneous pore and surface, and MV failed to be adsorbed by monolayer. The maximum adsorption capacity of FcPOP could be calculated from Langmuir model to be 516 mg/g. The value is much higher than that of active carbon or most common adsorbents, and but lower than that of functional porous polymers with carboxyl group, which has strong electrostatic interaction with cationic MV. Comparison of adsorption capacities of various adsorbents for MV is provided in Figure 7(a). The interaction between electron-rich groups in FcPOP and MV, homogeneous pore size distribution, and small size of MV molecules (11.3 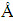 , Figure S11) could be responsible for excellent adsorption property of FcPOP.
, Figure S11) could be responsible for excellent adsorption property of FcPOP.

Figure 6 Experimental data of equilibrium adsorption isotherm, and fitting curves derived from Langmuir, Freundlich, and Redlich-Peterson models
Table 2 Parameters and correlation coefficient R2 of adsorption isotherm models


Figure 7 Comparison of adsorption capacities of various adsorbents for MV (a), and cyclic adsorption efficiency of MV onto FcPOP (b)
Cyclic regeneration and resuability are significantly important property of adsorbents. Cyclic adsorption efficiency of FcPOP was investigated with water/methanol co-solvent with NaCl as an eluent, which has proved excellent capacity to recover cationic dyes from porous polymers in our previous work [27]. Cyclic adsorption experiment was the same to that of adsorption kinetics. FcPOP were separated centrifugally, and then extracted with the eluent. Eight adsorption/desorption cycles were performed to examine MV removal efficiency of FcPOP, and the results are shown in Figure 7(b). Obviously, the adsorption capacity of FcPOP was only slightly decreased after 8 cycles, and the removal efficiency of MV still exceeded 96%, indicating an excellent stable adsorption capacity. A slightly declining of adsorption efficiency could be resulted by the progressive loss of pore structure during cyclic adsorption/desorption process. The high removal efficiency of dye, simple regeneration process, and excellent reusability of FcPOP in cyclic adsorption/desorption highlighted the prospective application in field of dyeing wastewater treatment.
4 Conclusions
Ferrocene-based porous organic polymer (FcPOP) was constructed by Schiff-base reaction of 1,1'-ferrocenedicarboxaldehyde and 5,10,15,20- tetrakis(4-aminophenyl)-21H,23H-porphine.FcPOP exhibited excellent thermal stability and high porosity. The incorporation of ferrocene blocks was favorable to strengthen the interaction with CO2, and improve the CO2 uptake and ideal selectivity. Excellent physicochemical stability, narrow pore size distribution, and highly electron- rich conjugated blocks prompted us to extend FcPOP application for removal of MV from aqueous solution. Nonlinear pesudo-second order model exhibited better fitting effect in comparison with pesudo-first order model. Investigation of adsorption isotherm indicated that main adsorption sites of FcPOP were the inhomogeneous pore and surface. Furthermore, the high removal efficiency of dye, simple regeneration process, and excellent reusability of FcPOP in cyclic adsorption/ desorption highlighted the prospective application in field of dyeing wastewater treatment.
Supporting information
Scheme S1 Synthesis route of 5,10,15,20-tetrakis(4-aminophenyl)-21H, 23H-porphine
S1 1H-NMR spectrum of 5,10,15,20-tetrakis(4- aminophenyl)-21H, 23H-porphine

Figure S1 1H-NMR spectrum of 5,10,15,20-tetrakis(4- aminophenyl)-21H, 23H-porphine
S2 FT-IR spectrum of FcPOP

Figure S2 FT-IR spectrum of FcPOP
S3 XRD pattern of FcPOP

Figure S3 XRD pattern of FcPOP
S4 TGA curve of FcPOP
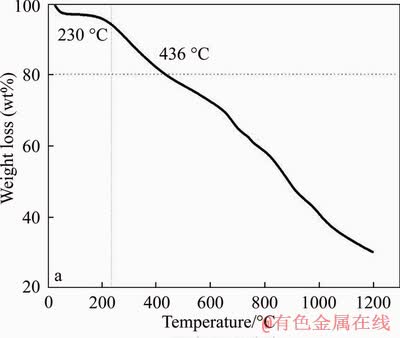
Figure S4 TGA curve of FcPOP
S5 BET transform plot
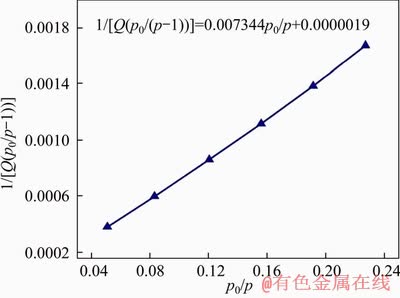
Figure S5 BET transform plot of FcPOP
S6 Qst simulation
The isosteric heat of adsorption was evaluated by the binding affinity single-component adsorption isotherms based on virial equation.
1) Virial equation
A virial-type expression in the following form can be used to fit the experimental isotherm data for a given material at different temperatures.
 (I)
(I)
where N is the amount adsorbed at a pressure P and a temperature T; m and n determine the number of terms which are required to adequately describe the isotherm; both ai and bi are temperature- independent empirical parameters. The isosteric heat of adsorption as a function of uptake was calculated by the virial coefficients from a0 to am:
 (II)
(II)
where R is the universal gas constant,8.314 J/(K·mol).
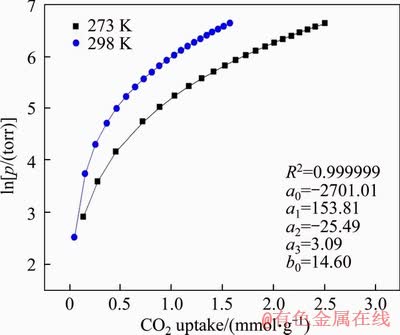
Figure S6 CO2 isotherms at 273 K and 298 K (symbols) and virial equation fits (lines) for FcPOP lnp= lnN+(-2701.01+153.81N-25.49N2+3.09N3)/273+14.6
S7 Selectivity of FcPOP

Figure S7 CH4 isotherms at 273 K and 298 K (symbols) and virial equation fits (lines) for FcPOP lnp=lnN+ (-1970.2094+181.7674N-60.8063N2)/273+13.8742
S8 Removal of MV from aqueous solution
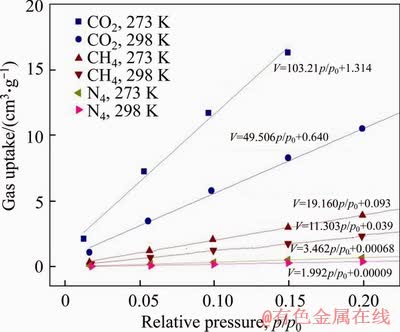
Figure S8 CO2/N2 and CO2/CH4 selectivities derived from IAST calculation at 273 K and 298 K (CO2(273 K):V=103.216p/p0+1.314; CO2 (298 K): V=49.506p/p0+ 0.640; CH4 (273 K): V=19.160p/p0+0.093; CH4 (298 K): V=11.303p/p0+0.039; N2 (273 K): V=3.462p/p0+0.00068 N2 (298 K): V=1.992p/p0+0.00009
Table S1 CO2 and CH4 uptake and selectivities of FcPOP at 273 K and 298 K
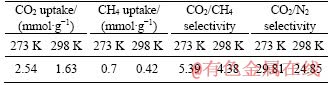
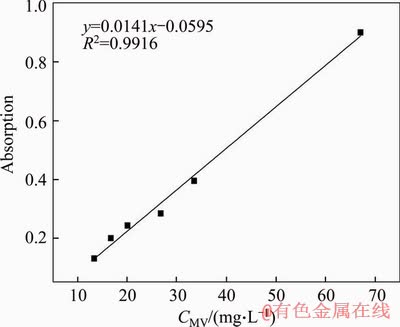
Figure S9 Linear fitting between MV concentration and UV absorption

Figure S10 UV absorption spectra of aqueous MV solution at an interval of 5 min
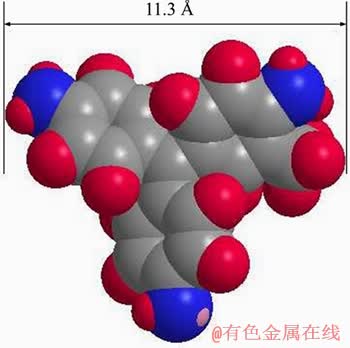
Figure S11 Molecular size of MV2B optimized by MM2
References
[1] WU S F, LIU Y, YU G P, GUAN J G, PAN C Y, DU Y, XIONG X, WANG Z G. Facile preparation of dibenzoheterocycle-functional nanoporous polymeric networks with high gas uptake capacities [J]. Macromolecules 2014, 47: 2875-2882. DOI: 10.1021/ ma500080s.
[2] TIAN Q H, WANG X Y, MAO F F, GUO X Y. Absorption performance of DMSA modified Fe3O4@SiO2core/shell magnetic nanocomposite for Pb2+removal [J]. Journal of Central South University, 2018, 25: 709-718. DOI: 10.1007/ s11771-018-3969-3
[3] LIU Y G, LI T T, ZENG G M, ZHENG B H, XU W H, LIU S B. Removal of Pb(Π) from aqueous solution by magnetic humic acid/chitosan composites [J]. Journal of Central South University, 2016, 23: 2809-2817. DOI: 10.1007/s11771-016- 3344-1.
[4] LINDEMANN P, TSOTSALAS S, SHISHATSKIY V, ABETZ P. KROLLA-SIDENSTEIN C, AZUCENA L, MONNEREAU A, BEYER A, GOLZHAUSER V, MUGNAINI H, GLEIMANN S, BRASES, WOLL C. Preparation of freestanding conjugated microporous polymer nanomembranes for gas separation [J]. Chemistry of Materials, 2014, 26: 7189-7193.
[5] ZHANG W J, TANG J T, YU W G, HUANG Q, FU Y, KUANG G C, PAN C Y, YU G P. Visible light-driven C-3 functionalization of indoles over conjugated microporous polymers [J]. ACS Catal, 2018, 8: 8084-8091. DOI: 10.1021/cm503924h.
[6] COLSON J W, WOLL A R, MUKHERJEE A, LEVENDORF M P, SPITLER E L, SHIELDS V B, SPENCER M G, PARK J, DICHTEL W R. Oriented 2D covalent organic framework thin films on single-layer graphene [J]. Science, 2011, 332: 228-231. DOI: 10.1126/ science.1202747.
[7] MCKEOWN N B, BUDD P M. Exploitation of intrinsic microporosity in polymer-based materials [J]. Macromolecules, 2010, 43: 5163-5176. DOI: 10.1021/ ma1006396.
[8] LIU Q Q, XIA B J, HUANG J, LIAO B, LIU H, OU B L, CHEN L, ZHOU Z Z. Hypercrosslinked polystyrene microspheres with ultra-high surface area and their application in gas storage [J]. Materials Chemistry and Physics, 2017, 199: 616-622. DOI: 10.1016/j.matchemphys. 2017.07.032.
[9] LI G, LIU Q Q, XIA B J, HUANG J, LI S Z, GUAN Y Z, ZHOU H, LIAO B, ZHOU Z Z, LU B. Synthesis of stable metal-containing porous organic polymers for gas storage [J]. European Polymer Journal, 2017, 91: 242-247. DOI: 10.1016/j.eurpolymj.2017.03.014.
[10] ZHENG B S, HUANG L, CAO X Y, SHEN S H, CAO H F, HANG C, ZENG W J, WANG Z X. A highly porous acylamide decorated MOF-505 analogue exhibiting large and selective CO2 gasuptake capability [J]. Cryst Eng Comm, 2018, 20: 1874-1881. DOI:
[11] WANG Z X, LUO X ZHENG B S, HUANG L, HANG C, JIAO Y C, CAO X Y, ZENG W J, YUN R R. Highly selective carbon dioxide capture and cooperative catalysis of a water-stable acylamide-functionalized metal-organic framework [J]. European Journal of Inorganic Chemistry, 2018, 11: 1309-1314. DOI: 10.1002/ ejic.201701404.
[12] DAWSON R, COOPER A I, ADAMS D J. Nanoporous organic polymer networks [J] Progress inPolymerScience, 2012, 37: 530-563. DOI: 10.1016/j.progpolymsci.2011.09. 002.
[13] LIU Q Q, TANG Z, OU B L, LIU L H, ZHOU Z H, SHEN S H, DUAN Y X. Design, preparation, and application of ordered porous polymer materials [J]. Materials Chemistry and Physics, 2014, 144: 213-225. DOI: 10.1016/ j.matchemphys.2014.01.013.
[14] XU Y H, JIN S B, XU H, NAGAI A, JIANG D L. Conjugated microporous polymers: Design, synthesis and application [J]. Chemical Society Reviews, 2013, 42: 8012-8031. DOI: 10.1039/C3CS60160A.
[15] KAUR P, HUPP J T, NGUYEN S T. Porous organic polymers in catalysis: Opportunities and challenges [J]. ACS Catalysis, 2011, 1: 819-835. DOI: 10.1021/cs200131g.
[16] SRINIVASU K, GHOSH S K. Hydrogen adsorption in lithium decorated conjugated microporous polymers: A DFT investigation [J]. RSC Advances, 2014, 4: 4170-4176. DOI: 10.1039/C3RA44942D.
[17] MA H, REN H, ZOU X, MENG S, SUN F X, ZHU G S. Post-metalation of porous aromatic frameworks for highly efficient carbon capture from CO2+N2 and CH4+N2 mixtures [J]. Polymer Chemistry, 2014, 5: 144-152. DOI: 10.1039/ C3PY00647F.
[18] JI G P, YANG Z Z, ZHAO Y F, ZHANG H Y, YU B, XU J L, XU H J, LIU Z M. Synthesis of metalloporphyrin-based conjugated microporous polymer spheres directed by bipyridine-type ligands [J]. Chemistry Communications, 2015, 51: 7352-7355. DOI: 10.1039/C5CC00609K.
[19] VAN STAVEREN D R, METZLER-NOLTE N. Bioorganometallic chemistry of ferrocene [J]. Chemical Reviews, 2004, 104: 5931-5985. DOI: 10.1021/cr0101510.
[20] LIU Q Q, TANG Z, WU M D, LIAO B, ZHOU H, OU B L, YU G P, ZHOU Z H, LI X J. Novel ferrocene-based nanoporous organic polymers for clean energy application [J]. RSC Advances, 2015, 5: 8933-8977. DOI: 10.1039/ c4ra12834f.
[21] FU X,ZHANG Y D, GU S, ZHU Y, YU G P, PAN C Y, WANG Z G, HU Y H. Metal microporous aromatic polymers with improved performance for small gas storage [J]. Chemistry–A European Journal, 2015, 21: 13357-13363. DOI: 10.1002/chem.201501594.
[22] LI Gen, LIU Qing-quan, LIAO Bo, CHEN Li-juan, ZHOU Hu, ZHOU Zhi-hua, XIA Bi-jiang, HUANG Jing, LIU Bin. Synthesis of novel ferrocene-based conjugated microporous polymers with intrinsic magnetism [J]. European Polymer Journal, 2017, 93: 556-560. DOI: 10.1016/j.eurpolymj. 2017.06.034.
[23] LIN J X, ZHAN S L, FANG M H, QIAN X Q. The adsorption of dyes from aqueous solution using diatomite [J]. Journal of Porous Materials, 2007, 14: 449-455. DOI: 10.1007/s10934-006-9039-5.
[24] LANGMUIR I. The constitution and fundamental properties of solids and liquids, Part I. Solids [J]. Journal of the American Chemical Society, 1916, 38: 2221-2295. DOI: 10.1021/ja02268a002.
[25] ZHOU Y, LIANG C, YU J, JIANG X. Adsorption properties of a novel 3D graphene/MgO composite for heavy metal ions [J]. Journal of Central South University,2019, 26: 813-823. DOI: 10.1007/s11771-019-4051-5.
[26] REDLIEH O, PETERSON D L. A useful adsorption isotherm [J]. Journal of Physical Chemistry, 1959, 63: 1024. DOI: 10.1021/j150576a611.
[27] LIU Q Q, PAN C Y. A novel route to treat wastewater containing cationic dyes [J]. Separation Science and Technology, 2012, 47: 630-635. DOI: 10.1080/01496395. 2011.618485.
[28] WOODBRIDGE C M, PUGMIRE D L, JOHNSON R C, BOAG N M, LANGELL M A. HREELS and XPS studies of ferrocene on Ag(100) [J]. The Journal of Physical Chemistry B, 2000,104: 3085-3093. DOI: 10.1021/jp993235+.
[29] ZHANG T, ZHOU F, HUANG J H, MAN R L. Ethylene glycol dimethacrylate modified hypercross-linked resins: Porogen effect on pore structure and adsorption performance [J]. Chemical Engineering Journal, 2018, 339: 278-287. DOI: 10.1016/j.cej.2018.01.138.
[30] GAO J, JAPIP S, CHUNG T S. Organic solvent resistant membranes made from a cross-linked functionalized polymer with intrinsic microporosity (PIM) containing thioamide groups [J]. Chemical Engineering Journal, 2018, 353: 689-698. DOI: 10.1016/j.cej.2018.07.156.
[31] SING K, EVERETT D, HAUL R, MOSCOU L, PIEROTTI R, ROUQUEROL J, SIEMIENIEWSKA T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity [J]. Pure and applied chemistry, 1985, 57: 603-619. DOI: 10.1351/pac198557040603.
[32] MOHANTY P, KULL LD, LANDSKRON K. Porous covalent electron-rich organonitridic frameworks as highly selective sorbents for methane and carbon dioxide [J]. Nature Communication, 2011, 2: 401-405. DOI: 10.1038/ ncomms1405.
[33] LIAO Y Z, WEBER J, FAUL C F J. Conjugated microporous polytriphenylamine networks [J]. Chemistry Communications, 2014, 50: 8002-8005. DOI: 10.1039/ C4CC03026E.
[34] COTE A P, BENIN A I, OCKWIG N W, O’KEEFFE M, MATZGER A J, YAGHI O M.Porous, cystalline, covalent organic frameworks [J]. Science, 2005, 310: 1166-1170. DOI: 10.1126/science.1120411.
[35] FURUKAWA H, YAGHI O M. Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications [J]. Journal of the American Chemical Society, 2009, 131: 8875-8883. DOI: 10.1021/ja9015765.
[36] YUAN R, REN H, YAN Z, WANG A, ZHU G S. Robust tri(4-ethynylphenyl)amine-based porous aromatic frameworks for carbon dioxide capture [J]. Polymer Chemistry, 2014, 5: 2266-2272. DOI: 10.1039/ C3PY01252B.
[37] YUAN Y, HUANG H, CHEN L, CHEN Y. N,N′-bicarbazole: A versatile building block toward the construction of conjugated porous polymers for CO2 capture and dyes adsorption [J]. Macromolecules, 2017, 50: 4993-5003. DOI: 10.1021/acs.macromol.7b00971.
[38] RABBANI M G, EL-KADERI H M. Synthesis and characterization of porous benzimidazole-linked polymers andtheir performance in small gas storage and selective uptake [J]. Chemistry of Materials, 2012, 24: 1511-1517. DOI: 10.1021/cm300407h.
[39] LAYBOURN A, DAWSON R, CLOWES R, IGGO J A, COOPER A I, KHIMYAK Y Z, ADAMS D J. Branching out with aminals: Microporous organic polymers from difunctional monomers [J]. Polymer Chemistry, 2012, 3: 533-537. DOI: 10.1039/C2PY00506A.
[40] YU M, WANG X, YANG X, ZHAO Y, JIANG J X. Conjugated microporous copolymer networks with enhanced gas adsorption [J]. Polymer Chemistry, 2015, 6: 3217-3233. DOI: 10.1039/C5PY00295H.
[41] WANG Y S, ZENG L, REN X F, SONG H, WANG A Q. Removal of methyl violet from aqueous solutions using poly(acrylic acid-co-acrylamide)/attapulgite composite [J]. Journal of Environmental Science, 2010, 22: 7-14. DOI: 10.1016/S1001-0742(09)60068-1.
[42] MITTAL H, KUMAR V, RAY S S. Adsorption of methyl violet from aqueous solution using gumxanthan/Fe3O4 based nanocomposite hydrogel [J]. International Journal of Biological Macromolecules, 2016, 89: 1-11. DOI: 10.1016/ j.ijbiomac.2016.04.050.
[43] BHATTACHARYYA R, RAY S K. Removal of congo red and methyl violet from water using nano clay filled composite hydrogels of poly acrylic acid and polyethylene glycol [J]. Chemical Engineering Journal, 2015, 260: 269-283. DOI: 10.1016/j.cej.2014.08.030.
[44] LI P, SU Y J, WANG Y, LIU B, SUN L M. Bioadsorption of methyl violet from aqueous solution onto Pu-erh tea powder [J]. Journal of Hazardous Materials, 2010, 179: 43-48. DOI: 10.1016/j.jhazmat. 2010.02.054.
[45] XU R K, XIAO S C, YUAN J H, ZHAO A Z. Adsorption of methyl violet from aqueous solutions by the biochars derived from crop residues [J]. Bioresource Technology, 2011, 102: 10293-10298. DOI: 10.1016/j.biortech.2011.08. 089.
[46] AZIZIAN S, HAERIFAR M, BASHIRI H. Adsorption of methyl violet onto granular activated carbon: Equilibrium, kinetics and modeling [J]. Chemical Engineering Journal, 2009, 146: 36-41. DOI: 10.1016/j.cej.2008.05.024.
[47] LIU R C, ZHANG B, MEI D D, ZHANG H Q, LIU J D. Adsorption of methyl violet from aqueous solution by halloysite nanotubes [J]. Desalination, 2011, 268: 111-116. DOI: 10.1016/j.desal.2010.10.006.
[48] OFOMAJA A E. Kinetic study and sorption mechanism of methylene blue and methylviolet onto mansonia (Mansoniaaltissima) wood sawdust [J]. Chemical Engineering Journal, 2008, 143: 85-95. DOI: 10.1016/j.cej. 2007.12.019.
(Edited by HE Yun-bin)
中文导读
用于气体捕获与甲基紫吸附的二茂铁基多孔有机聚合物
摘要:以二茂铁与卟啉衍生物为构筑单元,通过希夫碱偶合反应制备了二茂铁基多孔有机聚合物(FcPOP)。系统表征了FcPOP的结构与形貌,FcPOP表现出良好的热稳定性、高孔隙率、微孔结构以及均匀的孔径分布。二茂铁单元具有高度富电子特性,赋予FcPOP优秀的CO2和甲基紫吸附能力。甲基紫吸附动力学研究表明,FcPOP对甲基紫的吸附符合准二级动力学模型。以Langmuir等温线模型计算,得到FcPOP的最大吸能力达到516 mg/g。更重要的是,FcPOP可被简单再生,再重复应用于高效除去印染废水中的甲基紫。总的来说,制备的FcPOP在气体捕获和印染废水处理领域中表现出良好的应用前景。
关键词:二茂铁;有机多孔聚合物;气体捕获;印染废水
Foundation item: Project(51778226) supported by the National Natural Science Foundation of China; Project(2018JJ3159) supported by the Hunan Provincial Natural Science Foundation for Youths, China
Received date: 2019-04-01; Accepted date: 2020-02-14
Corresponding author: LIU Qing-quan, PhD, Professor; Tel: +86-731-58290782; E-mail:qqliu@hnust.edu.cn; ORCID: 0000-0003-3846- 4268

