采用硫化钠分离富集湿法炼锌渣中汞与硫
来源期刊:中国有色金属学报(英文版)2015年第2期
论文作者:王子阳 蔡晓兰 张泽彪 张利波 王仕兴 彭金辉
文章页码:640 - 646
Key words:flotation sulfur concentrate; sodium sulfide leaching; carbon dioxide precipitating; mercury enrichment; elemental sulfur recovery
摘 要:采用Na2S浸出-CO2沉淀法对浮选硫精矿中汞的分离和富集以及元素硫的回收进行研究。结果表明,在常温、Na2S浓度为1.5 mol/L、液固比为6:1、浸出时间为30 min的条件下,元素硫的浸出率可达98%以上,汞的平均富集率为98.13%,渣中汞含量为原矿石的5.23倍。向浸出液中通入CO2气体,充分搅拌溶液,在CO2流量为200 mL/min,通气时间为150 min的条件下,元素硫从溶液中析出,回收率可达到97.67%,获得的元素硫纯度为99.75%,符合GB/T2449—2006工业硫磺一等品标准。
Abstract: The separation and enrichment of mercury and the recovery of elemental sulfur from flotation sulfur concentrate in zinc pressure leaching process were carried out by sodium sulfide leaching and carbon dioxide precipitating. The results show that the leaching rate of elemental sulfur is more than 98%, and 98.13% of mercury is enriched in the residue, under the optimized conditions of sodium sulfide concentration 1.5 mol/L, liquid/solid ratio 6:1 and leaching time 30 min at room temperature. In addition, the content of mercury is enriched 5.23 times that in the leaching residue. The elemental sulfur is precipitated from leaching solution under conditions of carbon dioxide flow rate 200 mL/min and blowing time 150 min, while solution is stirred adequately. The recovery efficiency of elemental sulfur reaches 97.67%, and the purity of elemental sulfur is 99.75%, meeting the requirements of industrial first-rate product standard according to the national standard of GB/T 2449-2006 (PRC).
Trans. Nonferrous Met. Soc. China 25(2015) 640-646
Zi-yang WANG1, Xiao-lan CAI1, Ze-biao ZHANG1,2,3, Li-bo ZHANG1,2,3, Shi-xing WANG1,2,3, Jin-hui PENG1,2,3
1. Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China;
2. National Local Joint Engineering Laboratory of Engineering Applications of Microwave Energy and
Equipment Technology, Kunming University of Science and Technology, Kunming 650093, China;
3. Key Laboratory of Unconventional Metallurgy of Ministry of Education,
Kunming University of Science and Technology, Kunming 650093, China
Received 3 March 2014; accepted 14 July 2014
Abstract: The separation and enrichment of mercury and the recovery of elemental sulfur from flotation sulfur concentrate in zinc pressure leaching process were carried out by sodium sulfide leaching and carbon dioxide precipitating. The results show that the leaching rate of elemental sulfur is more than 98%, and 98.13% of mercury is enriched in the residue, under the optimized conditions of sodium sulfide concentration 1.5 mol/L, liquid/solid ratio 6:1 and leaching time 30 min at room temperature. In addition, the content of mercury is enriched 5.23 times that in the leaching residue. The elemental sulfur is precipitated from leaching solution under conditions of carbon dioxide flow rate 200 mL/min and blowing time 150 min, while solution is stirred adequately. The recovery efficiency of elemental sulfur reaches 97.67%, and the purity of elemental sulfur is 99.75%, meeting the requirements of industrial first-rate product standard according to the national standard of GB/T 2449-2006 (PRC).
Key words: flotation sulfur concentrate; sodium sulfide leaching; carbon dioxide precipitating; mercury enrichment; elemental sulfur recovery
1 Introduction
The solid wastes generated in the nonferrous metal smelting process usually contain toxic heavy metals. Preservation of these wastes not only causes enormous waste of resources, but also brings huge environment pressure. Since both mercury and zinc have the affinity to sulfur, element mercury is often associated in zinc ore, especially in zinc sulfide ore. Zinc smelting is widely regarded as one of the primary anthropogenic sources of mercury emission to the atmosphere [1,2]. Hundreds of tons of mercury are emitted to the environment in the process of zinc smelting in the world every year [2,3]. In 2003, mercury emission to atmosphere reaches 187.6 t from zinc smelting in China, which accounts for 27% of anthropogenic mercury emissions to atmosphere in China [4]. At present, some studies have been conducted on mercury emission to atmosphere and its environmental impacts from zinc smelting in China [5,6].
In recent years, great changes have occurred in the zinc smelting industry in China. Some new technologies, such as pressure leaching and atmospheric oxygen enrichment leaching, have been adopted. New technology will have more applications in the zinc smelting industry in the next few years [7]. The pressure leaching process does not need roasting pretreatment of ore, which avoids sulfur dioxide flue gas producing acid process. The removal of mercury needs to be carried out both from the flue gas containing mercury [8,9] and from acidic wastewater generated in sulfuric acid production [10–12]. In the pressure leaching process, mercury and sulfur mainly remain in the leaching residue in a stable form of mercury sulfide and elemental sulfur, respectively, which may reduce the environmental impact [13]. However, the elemental sulfur in the leaching residue, due to great blending with valuable metals of mercury and zinc and so forth, needs further treatment to achieve comprehensive utilization. The methods of elemental sulfur recovery from hydrometallurgical residue cover flotation, hot filtration, ammonium sulfide, tetrachloroethylene and so on [14-16], but these methods still have some problems.
The purpose of this study is to firstly leach flotation sulfur concentrate derived from hydrometallurgical zinc residue with sodium sulfide, and the elemental sulfur is dissolved into the leaching solution while mercury in a stable form is enriched into the leaching residue. Then, the elemental sulfur is recovered when carbon dioxide is bubbled into the leaching solution. The sodium sulfide leaching-carbon dioxide precipitating method may make reagents recycle back into process and obtain high quality sulfur, with easy operation and simple equipments.
2 Chemical reactions and thermodynamics
The elemental sulfur reacts with sodium sulfide (Na2S) in the solution to form a polysulfide according to the following reaction [14,15]:
Na2S+(x-1)S0=Na2Sx (1)
Meanwhile, the soluble mercury cation (Hg2+) from flotation sulfur concentrate can also react with sulfidion (S2-) from the sodium sulfide solution, generating the mercuric sulfide deposit with tiny solubility product constant Ksp of 4.0×10-53, so the element mercury is still left in the residue. The reaction is as follows:
Hg2++S2-=HgS↓ (2)
When the elemental sulfur from material is dissolved, the solution is then filtered to produce an essentially sulfur free residue. The polysulfide anion is fairly unstable, and the polysulfide-rich filtrate obtained is decomposed to recover sulfur under an acid environment when it is treated with carbon dioxide (CO2) through the following reaction:
Na2Sx+CO2+H2O=NaHS+NaHCO3+(x-1)S0 (3)
The elemental sulfur is recovered through a second filtration of the mixture solution, and is further dried to sulfur product. The mixture solution of sodium bisulfide and bicarbonate can be regenerated to sodium sulfide by the use of lime (CaO) according to the following reaction:
NaHS+NaHCO3+CaO=Na2S+CaCO3+H2O (4)
The regenerated sodium sulfide solution is recycled back into the initial leaching process, while the solid limestone (CaCO3) can be decomposed by roasting to carbon dioxide and lime (CO2 + CaO) which may be reused back into the reaction (3) and the reaction (4), respectively.
Thermodynamic analysis includes the calculations of standard Gibbs free energy (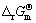 ) and standard equilibrium constant (KΘ). The reaction (1) can be simplified to the following reaction:
) and standard equilibrium constant (KΘ). The reaction (1) can be simplified to the following reaction:
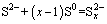 (5)
(5)
The x of polysulfide ion (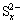 ) varies from 2 to 5 [17]. Standard Gibbs free energies of formation (
) varies from 2 to 5 [17]. Standard Gibbs free energies of formation (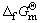 ) of different sulfur ions at 298.15 K [17,18] are shown in Table 1. The Gibbs free energy and standard equilibrium constant for reactions of dissolving sulfur were calculated at 298.15 K and the values are shown in Table 2. The negative values of the standard Gibbs free energy,
) of different sulfur ions at 298.15 K [17,18] are shown in Table 1. The Gibbs free energy and standard equilibrium constant for reactions of dissolving sulfur were calculated at 298.15 K and the values are shown in Table 2. The negative values of the standard Gibbs free energy, 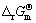 , for the reactions (8) and (9), indicate that they are all thermodynamically feasible at atmospheric pressure and temperature 298.15 K, and formations of
, for the reactions (8) and (9), indicate that they are all thermodynamically feasible at atmospheric pressure and temperature 298.15 K, and formations of 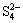 and
and  have higher thermodynamic probability. The large values of standard equilibrium constant, KΘ, for the reactions (8) and (9), further suggest probability of formations of
have higher thermodynamic probability. The large values of standard equilibrium constant, KΘ, for the reactions (8) and (9), further suggest probability of formations of 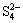 and
and  . Based on the above thermodynamic analysis, sulfur dissolution in sodium sulfide solution can occur spontaneously at atmospheric pressure and room temperature.
. Based on the above thermodynamic analysis, sulfur dissolution in sodium sulfide solution can occur spontaneously at atmospheric pressure and room temperature.
Table 1 Standard Gibbs free energy of formation (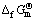 ) of different sulfur ions at 298.15 K
) of different sulfur ions at 298.15 K
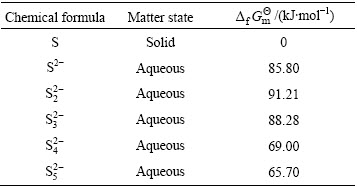
Table 2 Values of standard Gibbs free energy (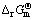 ) and standard equilibrium constant (KΘ) at 298.15 K for reactions of dissolving sulfur
) and standard equilibrium constant (KΘ) at 298.15 K for reactions of dissolving sulfur
3 Experimental
3.1 Materials
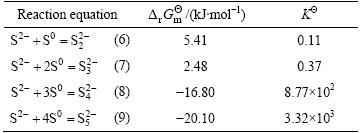
The raw material for this study is flotation sulfur concentrate from hydrometallurgical residue during the zinc pressure leaching process. The chemical composition of flotation sulfur concentrate is shown in Table 3. It can be seen that the amount of elemental sulfur is 84.98%, almost accounting for 94.89% of total sulfur. In addition, mercury content is as high as 951.7 g/t. The X-ray diffraction (XRD) pattern of flotation sulfur concentrate is shown in Fig. 1. It indicates that the major mineral in flotation sulfur concentrate is elemental sulfur in the form of S8, and other minerals include sphalerite (ZnS) and pyrite (FeS2). During the zinc pressure leaching process, the minerals of mercury in residue mainly exist as mercuric sulfide (HgS), and probably include a little soluble mercuric sulfate (HgSO4) and mercuric chloride (HgCl2).
Table 3 Chemical composition of flotation sulfur concentrate

Fig. 1 XRD pattern of flotation sulfur concentrate
The main reagents used are sodium sulfide nonahydrate (Na2S·9H2O) (AR), industrial grade CO2 and calcium oxide (CaO) (AR).
3.2 Method
The flotation sulfur concentrate and the sodium sulfide solution were placed together in a reaction conical flask, and then leached under vigorous agitation. The leaching solution was filtered to get a leaching residue and a polysulfide filtrate. The leaching residue was dried at 60 °C and then conducted the weighing as well as elemental analysis. Besides, the sodium polysulfide filtrate placed in a homemade reaction vessel was appropriately stirred and simultaneously acidized with carbon dioxide. The elemental sulfur precipitated from the solution was subjected to filtration and drying, and sulfur product was finally obtained. The mixture solution of sodium bisulfide and bicarbonate, neutralized by lime, could be regenerated to sodium sulfide, which was recycled back into the initial leaching process. The flow sheet of flotation sulfur concentrate treated by sodium sulfide leaching-carbon dioxide precipitating method is shown in Fig. 2.
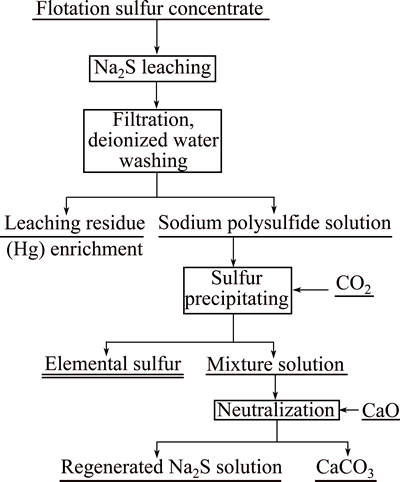
Fig. 2 Flow sheet of flotation sulfur concentrate treated by Na2S leaching-CO2 precipitating method
In this work, determination of the content of elemental sulfur in ore or leaching residue was carried out by a tube furnace combustion method. The sulfur product was determined by using the national standard of GB/T 2449—2006 (PRC). The content of mercury was analyzed by inductively coupled plasma atomic emission spectrometry (ICP-AES).
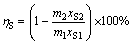 (10)
(10)
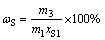 (11)
(11)
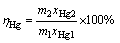 (12)
(12)
where ηS is leaching rate of elemental sulfur; ωS is recovery rate of elemental sulfur; ηHg is mercury enrichment rate in leaching residue, representing the efficiency of mercury recovery in leaching residue; m1, m2 and m3 are the mass of flotation sulfur concentrate, leaching residue and sulfur product, respectively; xS1 and xS2 are the mass fraction of elemental sulfur in flotation sulfur concentrate and leaching residue, respectively; xHg1 and xHg2 are the mass fraction of mercury in flotation sulfur concentrate and leaching residue, respectively.
4 Results and discussion
4.1 Sodium sulfide leaching process
4.1.1 Effect of liquid/solid ratio
The effect of liquid/solid ratio on sulfur leaching rate and mercury enrichment rate was investigated under conditions of flotation sulfur concentrate mass 50 g, sodium sulfide concentration 1.5 mol/L and leaching time 20 min at room temperature. The results are shown in Fig. 3.
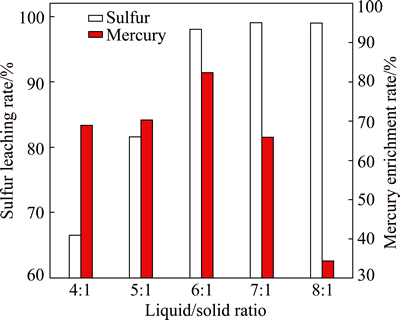
Fig. 3 Effect of liquid/solid ratio on leaching of sulfur and mercury
Figure 3 shows that the leaching rate of elemental sulfur increases greatly with increasing the amount of sodium sulfide. When the liquid/solid ratio is over 6:1, the curve of leaching sulfur changes to be steady. As for mercury, the enrichment rate of mercury first increases and then decreases with increasing liquid/solid ratio. At a low liquid/solid ratio, elemental sulfur cannot be leached completely. With increasing liquid/solid ratio, although sulfidion (S2-) concentration is constant, the amount still increases, which makes sulfur leach into solution and mercury enrich into residue gradually. However, when the liquid/solid ratio is over 6:1, sulfur dissolution in the sodium sulfide is basically saturated. By contrast, excessive sulfidion (S2-) can react further with mercuric sulfide deposit (HgS) to form soluble complexation anion [HgS2]2- [19,20], resulting in the decrease of enrichment rate of mercury in residue. So, comprehensively considering sulfur leaching rate and mercury enrichment rate, the suitable condition of liquid/solid ratio is 6:1.
4.1.2 Effect of sodium sulfide concentration
The effect of sodium sulfide concentration on the dissolution of sulfur and the enrichment of mercury, under the following conditions of flotation sulfur concentrate mass 50 g, liquid/solid ratio 6:1 and leaching time 20 min at room temperature, is shown in Fig. 4.
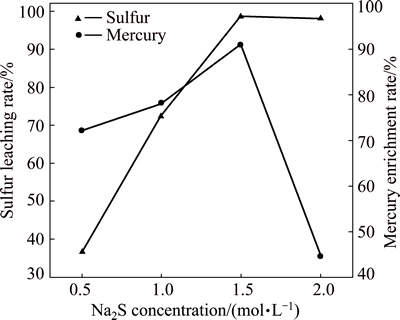
Fig. 4 Effect of sodium sulfide concentration on leaching of sulfur and mercury
The sodium sulfide concentration has a significant influence on the leaching process. It can be seen that the leaching rate of elemental sulfur increases dramatically before sodium sulfide concentration increases to 1.5 mol/L. After that, the leaching rate has no significant change. The enrichment rate of mercury increases as sodium sulfide concentration increases, but when concentration is over 1.5 mol/L, mercury enrichment rate sharply declines. Due to the fact that sodium sulfide concentration is too high, elemental sulfur is dissolved to reach saturation. The excessive sulfidion, however, can cause mercuric sulfide deposit to dissolve in the solution. In addition, when the concentration of sulfidion reaches a certain level, total polysulfide anion concentration in the solution does not basically change. With increasing sulfidion concentration, the average chain length x in polysulfide anion, 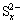 , changes, or the composition proportion of certain chain length
, changes, or the composition proportion of certain chain length 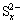 (x>1) raises [21]. The formation of certain chain length
(x>1) raises [21]. The formation of certain chain length 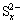 greatly accelerates the dissolution of HgS deposit, forming soluble mercuric polysulfide complexation anion [Hg(Sx)2]2- and [HgSxOH]-, and the dissolution is more significant especially in higher pH solution [22,23].
greatly accelerates the dissolution of HgS deposit, forming soluble mercuric polysulfide complexation anion [Hg(Sx)2]2- and [HgSxOH]-, and the dissolution is more significant especially in higher pH solution [22,23].
4.1.3 Effect of leaching temperature
The effect of leaching temperature on leaching process of flotation sulfur concentrate was investigated under conditions of flotation sulfur concentrate mass 50 g, sodium sulfide concentration 1.5 mol/L, liquid/solid ratio 6:1 and leaching time 20 min.
Figure 5 shows that the leaching rate of elemental sulfur and the enrichment rate of mercury both change steadily as leaching temperature ranges from 20 °C to 60 °C. The influence of leaching temperature on leaching effectiveness of flotation sulfur concentrate by sodium sulfide is thought to be little. Mercury, well separated from elemental sulfur, is effectively enriched in the residue. So, leaching process can be conducted at room temperature.
4.1.4 Effect of leaching time
The effect of leaching time on the degree of sulfur leaching and mercury enrichment was investigated under conditions of flotation sulfur concentrate mass 50 g, sodium sulfide concentration 1.5 mol/L and liquid/solid ratio 6:1 at room temperature (Fig. 6).
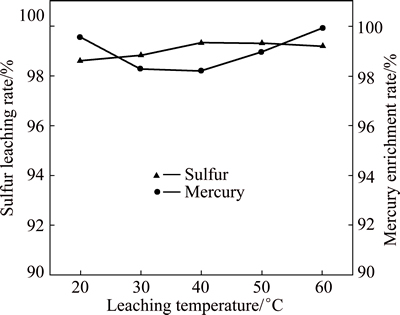
Fig. 5 Effect of leaching temperature on leaching of sulfur and mercury
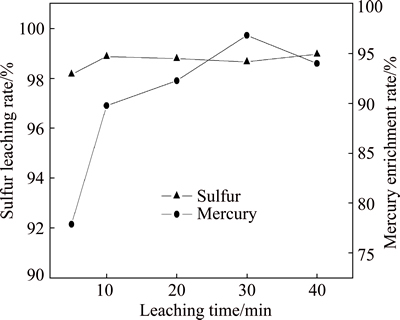
Fig. 6 Effect of leaching time on leaching of sulfur and mercury
As shown in Fig. 6, sodium sulfide is an effective reagent for dissolving elemental sulfur. The leaching rate of elemental sulfur is above 98% when leaching process is conducted only in 5 min. Later, the curve of leaching rate changes to be steady. With increasing leaching time, the enrichment rate of mercury first increases and then decreases, and is maximal at 30 min. The influence of leaching time on mercury enrichment rate is more significant than that on sulfur leaching rate. It can be conducted that 30 min is the suitable leaching time.
4.2 Precipitation of elemental sulfur by carbon dioxide
4.2.1 Effect of carbon dioxide flow rate and blowing time
Elemental sulfur is dissolved from flotation sulfur concentrate into polysulfide-rich leaching solution, which is subsequently blown with carbon dioxide. When pH of leaching solution reduces to a certain degree, polysulfide is decomposed and converted to elemental sulfur. The results of elemental sulfur precipitated from solution without stirring are shown in Fig. 7. The recovery rate of elemental sulfur gradually increases with increasing carbon dioxide flow rate and blowing time. However, the recovery efficiency is very low when the solution has no agitation. Only 72.44% of elemental sulfur from solution is recovered at carbon dioxide flow rate of 1000 mL/min and blowing time of 180 min.

Fig. 7 Recovery rate of elemental sulfur precipitated by carbon dioxide at different gas flow rates and blowing time
4.2.2 Effect of stirring
The influence of stirring on recovery rate of elemental sulfur is shown in Fig. 8. The results indicate that the stirring plays a critical role in the elemental sulfur recovery. The recovery rate is greatly improved when the solution is agitated appropriately. The recovery rate of elemental sulfur almost linearly increases as the blowing time increases at carbon dioxide flow rates of 200 and 400 mL/min, respectively, but a significant slowing down of the recovery rate in the final stage of blowing is noted. The recovery efficiency of elemental sulfur is 97.65% at carbon dioxide flow rate of 200 mL/min and blowing time 150 min. When the elemental sulfur is precipitated from polysulfide solution under appropriate stirring, carbon dioxide gas blew is divided into more tiny bubbles under the action of stirring, enlarging the reaction area of gas and liquid. On the other hand, tiny bubbles are revolved along with the solution to prolong the gas-liquid contact time. So, stirring provides a favorable condition for gas-liquid reaction kinetics.

Fig. 8 Recovery rate of elemental sulfur from solution under stirring at carbon dioxide flow rate of 200 and 400 mL/min, respectively
4.3 Validation test
In order to determine the process parameters according to the above analyses, comprehensive validation test was carried out when flotation sulfur concentrate was leached by sodium sulfide under contidions of sodium sulfide concentration 1.5 mol/L, liquid/solid ratio 6:1 and leaching time 30 min at room temperature, and elemental sulfur was precipitated from solution under stirring at carbon dioxide flow rate 200 mL/min and blowing time 150 min. The results are shown in Table 4.
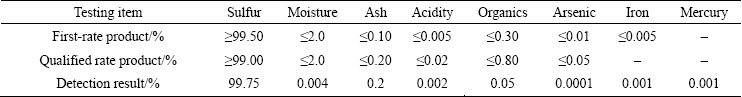
Table 4 shows that when flotation sulfur concentrate is treated by Na2S leaching-CO2 precipitating method, the average residue rate is 18.78%, and the content of elemental sulfur in leaching residue is quite slow, which indicates that sulfur dissolution efficiency is high. The average leaching rate and recovery rate of elemental sulfur are 98.43% and 97.67%, respectively. Besides, 98.13% of mercury is enriched into leaching residue, the average content of mercury reaches 4993 g/t in the residue, and is 5.23 times that in the ore. The mercury- containing residue is a high-grade mercury concentrate, and can be treated to further recover mercury. Detection results of elemental sulfur obtained in terms of the national standard of GB/T 2449—2006 (PRC) are shown in Table 5. The purity of elemental sulfur is 99.75%, and the content of mercury in the sulfur is 10 g/t, which has no influence on the sulfur product. The phases identified by XRD in leaching residue are mainly sphalerite and very few elemental sulfur (Fig. 9), which further confirms that elemental sulfur is effectively leached and valuable metals are enriched into the residue during leaching process.

Fig. 9 XRD pattern of leaching residue
Table 4 Results of validation test
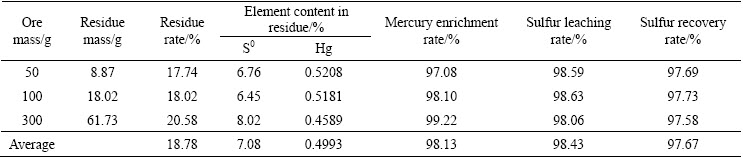
Table 5 Results of elemental sulfur obtained in terms of GB/T 2449—2006 (PRC)
5 Conclusions
1) The recovery of elemental sulfur and the separation and enrichment of mercury from flotation sulfur concentrate derived from zinc pressure leaching residue are feasible by sodium sulfide leaching-carbon dioxide precipitating.
2) The optimal experiment parameters are: sodium sulfide concentration 1.5 mol/L, liquid/solid ratio 6:1, leaching time 30 min and room temperature. The leaching rate of elemental sulfur is more than 98%, and 98.13% of mercury is enriched in leaching residue. The content of mercury in leaching residue is 5.23 times that in flotation sulfur concentrate.
3) The elemental sulfur is deposited from leaching solution at carbon dioxide flow rate 200 mL/min and blowing time 150 min while leaching solution is stirred adequately. The recovery rate of elemental sulfur is 97.67% with the purity 99.75%.
References
[1] Pacyna E G, Pacyna J M, Sundseth K, MUNTHE J, KINDBOM K, WILSON S, STEENHUISEN F, MAXSON P. Global emission of mercury to the atmosphere from anthropogenic sources in 2005 and projections to 2020 [J]. Atmospheric Environment, 2010, 44(20): 2487-2499.
[2] Nriagu J O, Pacyna J M. Quantitative assessment of worldwide contamination of air, water and soil by trace metals [J]. Nature, 1988, 333: 134-139.
[3] Pacyna E G, Pacyna J M, Steenhuisen F, WILSON S. Global anthropogenic mercury emission inventory for 2000 [J]. Atmospheric Environment, 2006. 40(22): 4048-4063.
[4] WU Ye, Wang Shu-xiao, Streets D G, HAO Ji-ming, CHAN M, JIANG Jing-kun. Trends in anthropogenic mercury emissions in China from 1995 to 2003 [J]. Environmental Science & Technology, 2006, 40(17): 5312-5318.
[5] LI Guang-hui, FENG Xin-bin, LI Zhong-gen, QIU Guang-le, SHANG Li-hai, LIANG Peng, WANG Ding-yong, YANG Yong-kui. Mercury emission to atmosphere from primary Zn production in China [J]. Science of the Total Environment, 2010, 408(20): 4607-4612.
[6] LI Guang-hui, FENG Xin-bin, QIU Guang-le, BI Xiang-yang, LI Zhong-gen, ZHANG Cheng, WANG Ding-yong, SHANG Li-hai, GUO Yan-na. Environmental mercury contamination of an artisanal zinc smelting area in Weining County, Guizhou, China [J]. Environmental Pollution, 2008, 154(1): 21-31.
[7] LIU San-ping, WANG Hai-bei, JIANG Kai-xi, ZHANG Bang-sheng. New development of zinc hydrometallurgy in China [J]. Mining & Metallurgy, 2009, 18(4): 25-27, 31. (in Chinese)
[8] HU Ze-ya. Production and expansion practice of Zhuzhou Smelter’s zinc I sulphuric acid system [J]. Sulphuric Acid Industry, 2008(5): 29-32. (in Chinese)
[9] TANG Guan-hua. Application of iodine complex-electrolytic method of removing mercury in sulfuric acid production [J]. Nonferrous Metals Engineering & Research, 2010, 31(3): 23-25. (in Chinese)
[10] Shigehiro K, Hiroyuki M, Masahiro I, koji t, takaki k. Selective removal of mercury(II) from wastewater using polythioamides [J]. Journal of Hazardous Materials, 2010, 175(1-3): 1113-1115.
[11] CHAI Li-yuan, WANG Qing-wei, WANG Yun-yan, LI Qing-zhu, YANG Zhi-hui, SHU Yu-de. Thermodynamic study on reaction path of Hg(II) with S(II) in solution [J]. Journal of Central South University of Technology, 2010, 17(2): 289-294. (in Chinese)
[12] WANG Qing-wei, CHAI Li-yuan, WANG Yun-yan, LI Qing-zhu. Novel technology for treatment of acidic wastewater containing Hg by biologics in zinc smelter [J]. The Chinese Journal of Nonferrous Metals, 2008, 18(s1): 416-421. (in Chinese)
[13] XU Zhi-feng, QIU Ding-fan, LU Hui-min, WANG Hai-bei. Review on research of oxidic-acidic pressure of zinc concentrates [J]. Nonferrous Metals, 2005, 57(2): 101-105. (in Chinese)
[14] Halfyard J E, Hawboldt K. Separation of elemental sulfur from hydrometallurgical residue: A review [J]. Hydrometallurgy, 2011, 109(1-2): 80-89.
[15] SUN Pei-mei, WEI Dai-jin, LI Hong-gui, ZHAO Zhong-wei, HUO Guang-sheng, LI Yun-jiao. Separation and enrichment of valuable elements from copper residue leached by chlorine [J]. Journal of Central South University, 2005, 36(1): 38-43. (in Chinese)
[16] PENG Peng, XIE Hui-qin, LU Li-zhu. Leaching of a sphalerite concentrate with H2SO4-HNO3 solutions in the presence of C2Cl4 [J]. Hydrometallurgy, 2005, 80(4): 265-271.
[17] QIN Yi-hong, ZHANG Li. Thermodynamic analysis of SO2-Na2S- H2O system [J]. Chemical Engineering (China), 2011, 39(3): 50-53. (in Chinese)
[18] LIN Ping-di. Inorganic chemical thermodynamics [M]. Beijing: Beijing Normal University Press, 1986. (in Chinese)
[19] TANG Ning, CHAI Li-yuan, MIN Xiao-bo. Research development in the treatment of mercury-containing wastewater [J]. Industrial Water Treatment, 2004, 24(8): 5-8, 13. (in Chinese)
[20] HUANG Ming-rong, GAO Guo-yu, HE Xiao-di. Research method of treatment of mercury-containing wastewater [J]. Chemical Engineering Design, 2010, 20(2): 33-35. (in Chinese)
[21] ZHU Guo-cai, FANG Zhao-heng, CHEN Jia-yong. A study on the leaching of gold from sulfide concentrates [J]. Precious Metals, 1994, 15(2): 26-31. (in Chinese)
[22] Paquette K E, Helz G R. Inorganic speciation of mercury in sulfidic waters: The importance of zero-valent sulfur [J]. Environmental Science & Technology, 1997, 31(7): 2148-2153.
[23] Jay J A, Morel F M M, Hemond H F. Mercury speciation in the presence of polysulfides [J]. Environmental Science & Technology, 2000, 34(11): 2196-2200.
王子阳1,蔡晓兰1,张泽彪1,2,3,张利波1,2,3,王仕兴1,2,3,彭金辉1,2,3
1. 昆明理工大学 冶金与能源工程学院,昆明 650093;
2. 昆明理工大学 微波能工程应用及装备技术国家地方联合工程实验室,昆明 650093;
3. 昆明理工大学 非常规冶金教育部重点实验室,昆明 650093
摘 要:采用Na2S浸出-CO2沉淀法对浮选硫精矿中汞的分离和富集以及元素硫的回收进行研究。结果表明,在常温、Na2S浓度为1.5 mol/L、液固比为6:1、浸出时间为30 min的条件下,元素硫的浸出率可达98%以上,汞的平均富集率为98.13%,渣中汞含量为原矿石的5.23倍。向浸出液中通入CO2气体,充分搅拌溶液,在CO2流量为200 mL/min,通气时间为150 min的条件下,元素硫从溶液中析出,回收率可达到97.67%,获得的元素硫纯度为99.75%,符合GB/T2449—2006工业硫磺一等品标准。
关键词:浮选硫精矿;硫化钠浸出;二氧化碳沉淀;汞富集;元素硫回收
(Edited by Xiang-qun LI)
Corresponding author: Xiao-lan CAI; Tel: +86-13708722098; Fax: +86-871-65189592; E-mail: cxl9761@163.com
DOI: 10.1016/S1003-6326(15)63647-0

