脱硅产物在铝酸钠溶液中常压析出的矿相转变
来源期刊:中国有色金属学报(英文版)2018年第2期
论文作者:蒋涛 潘晓林 吴艳 于海燕 涂赣峰
文章页码:367 - 375
关键词:拜耳法;脱硅;铝酸钠溶液;沸石;方钠石
Key words:Bayer process; desilication; sodium aluminate solution; zeolite; sodalite
摘 要:研究在95 °C下合成过饱和二氧化硅铝酸钠溶液时不同脱硅条件对脱硅产物的生成量、物相组成及其结晶度和显微组织的影响规律。在常压脱硅条件下,脱硅产物由无定型沸石、A型沸石、沸石和方钠石组成;脱硅产物的生成量和结晶度随着初始二氧化硅浓度、分子比和脱硅时间的增加而增加。降低初始二氧化硅浓度和分子比,增加脱硅时间,能够降低A型沸石的含量。沸石和方钠石是稳定的脱硅产物,而A型沸石只在高饱和二氧化硅浓度的铝酸钠溶液中析出。脱硅产物析出后均发生附聚,但不同类型脱硅产物的显微形貌明显不同;脱硅产物中Na2O与Al2O3摩尔比、SiO2与Al2O3摩尔比随着脱硅时间的增加而增大,从而使脱硅产物的晶胞体积增大。常压下过饱和二氧化硅铝酸钠溶液中脱硅产物的析出顺序为:无定型沸石→A型沸石→沸石→方钠石。
Abstract: The liquor concentration, mineral proportion, crystal parameters and micro morphology of various desilication products (DSPs) precipitated in silica-supersaturated sodium aluminate solution at 95 °C under different reaction conditions were systematically researched. The DSPs formed under atmospheric pressure comprise amorphous phase, zeolite A, zeolite and sodalite, and the DSPs concentration and crystallinity increase with the increase of initial silica concentration, initial molar ratio of caustic Na2O to Al2O3 (αK) and desilication duration. Decreasing the initial silica concentration, initial αK and increasing the desilication duration can reduce the proportion of zeolite A. The zeolite and sodalite are the stable DSPs, while the precipitation of zeolite A occurs at a high silica-supersaturated state in sodium aluminate solution. The DSPs are precipitated in the form of agglomerates, but the morphologies of various DSPs are quite different. Both the molar ratios of Na2O to Al2O3 and SiO2 to Al2O3 in DSPs increase with the increasing desilication duration, resulting in the increase of the cell volumes of various DSPs. The precipitation sequence of DSPs under atmospheric pressure is: amorphous phase→zeolite A→zeolite→sodalite.
Trans. Nonferrous Met. Soc. China 28(2018) 367-375
Tao JIANG, Xiao-lin PAN, Yan WU, Hai-yan YU, Gan-feng TU
School of Metallurgy, Northeastern University, Shenyang 110819, China
Received 18 November 2016; accepted 11 April 2017
Abstract: The liquor concentration, mineral proportion, crystal parameters and micro morphology of various desilication products (DSPs) precipitated in silica-supersaturated sodium aluminate solution at 95 °C under different reaction conditions were systematically researched. The DSPs formed under atmospheric pressure comprise amorphous phase, zeolite A, zeolite and sodalite, and the DSPs concentration and crystallinity increase with the increase of initial silica concentration, initial molar ratio of caustic Na2O to Al2O3 (αK) and desilication duration. Decreasing the initial silica concentration, initial αK and increasing the desilication duration can reduce the proportion of zeolite A. The zeolite and sodalite are the stable DSPs, while the precipitation of zeolite A occurs at a high silica-supersaturated state in sodium aluminate solution. The DSPs are precipitated in the form of agglomerates, but the morphologies of various DSPs are quite different. Both the molar ratios of Na2O to Al2O3 and SiO2 to Al2O3 in DSPs increase with the increasing desilication duration, resulting in the increase of the cell volumes of various DSPs. The precipitation sequence of DSPs under atmospheric pressure is: amorphous phase→zeolite A→zeolite→sodalite.
Key words: Bayer process; desilication; sodium aluminate solution; zeolite; sodalite
1 Introduction
The bauxite ores for the alumina extraction mainly comprise Al-, Si-, Fe-, Ti-containing minerals as well as lots of trace element minerals [1]. Among all the impurity minerals, the Si-containing minerals are considered to be the most harmful during the Bayer digestion process, in which the dissolution reactions of Si-containing minerals and the precipitation reactions of desilication products (DSPs) usually occur simulta- neously [2,3]. The precipitation of DSPs not only results in the losses of soda and alumina into the bauxite residue and the scaling on the reactor surfaces decreasing the heat transfer efficiency, but also increases the residue output deteriorating the settling property of digested slurry [4].
The DSPs consist of several types, such as zeolite (ZEO) series, sodalite (SOD) series, cancrinite (CAN) series, and hydrogarnet (HG) series [5,6]. The series of zeolite and sodalite have the same cubic sodalite- structure, while the cancrinite has a hexagonal structure. The above three series of DSPs are usually expressed by a generalized formula of Na8(Al6Si6O24)·X2·yH2O, where X=Cl-, OH-, 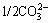 ,
, 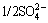 ,
, 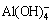 [7]. The series of hydrogarnet containing calcium, i.e., conventional hydrogarnet (3CaO·Al2O3·mSiO2·nH2O), andradite hydrogarnet (3CaO·Fe2O3·mSiO2·nH2O) and andradite-grossular hydrogarnet (Ca3(Al,Fe)2(SiO4)n- (OH)(12–4n)), form during the high pressure digestion with lime addition [8,9].
[7]. The series of hydrogarnet containing calcium, i.e., conventional hydrogarnet (3CaO·Al2O3·mSiO2·nH2O), andradite hydrogarnet (3CaO·Fe2O3·mSiO2·nH2O) and andradite-grossular hydrogarnet (Ca3(Al,Fe)2(SiO4)n- (OH)(12–4n)), form during the high pressure digestion with lime addition [8,9].
The desilication reactions during the Bayer process are usually complicated and diversified, which involve in three different processes, i.e., the pre-desilication process before digestion, the digestion process, and the diluted desilication process after digestion [10]. The pre- desilication process and the diluted desilication process belong to the atmospheric pressure desilication, which are aimed to dissolve the Si-containing minerals at a lower temperature to decrease the following scaling during digestion and to obtain a low silica concentration for the following gibbsite precipitation respectively. The digestion process belongs to the moderate or high pressure desilication depending on the bauxite type. The most researches about the desilication process were focused on the pressurized desilication during the Bayer digestion process in the past decades [11], especially for the kinetics and mechanisms of scale formation [12,13]. For example, WHITTINGTON et al [7] studied the composition of sodalite and cancrinite formed at 150-250 °C in sodium aluminate solution with different 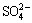 ,
, 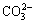 and Cl-, and found the magnitude of anion incorporation into the DSPs; BARNES et al [14,15] studied the desilication kinetics of spent Bayer liquor with unseeded or seeded sodalite crystals, and the mechanism of sodalite-to-cancrinite phase trans- formation; KAWASHIMA et al [16] studied the formation mechanism of several scales in single-stream Bayer plant heat exchangers; ARMSTRONG and DANN [17] compared the formation behavior of synthetic sodium aluminosilicate scale with Bayer refinery plant scale, and found that the large concentration of organic ions in Bayer liquor has little effect on the type of forming scales.
and Cl-, and found the magnitude of anion incorporation into the DSPs; BARNES et al [14,15] studied the desilication kinetics of spent Bayer liquor with unseeded or seeded sodalite crystals, and the mechanism of sodalite-to-cancrinite phase trans- formation; KAWASHIMA et al [16] studied the formation mechanism of several scales in single-stream Bayer plant heat exchangers; ARMSTRONG and DANN [17] compared the formation behavior of synthetic sodium aluminosilicate scale with Bayer refinery plant scale, and found that the large concentration of organic ions in Bayer liquor has little effect on the type of forming scales.
However, the researches focused on the atmospheric pressure desilication rarely appeared. Meanwhile, as the bauxite compositions are complex especially for the Si-containing minerals, it is usually difficult to study the desilication process in depth, especially for the precise crystal structure and morphology of DSPs [18]. In this work, the aim is to systematically investigate the precipitation behavior and mineral transition of DSPs in synthetic sodium aluminate solution under atmospheric pressure, which will provide theoretical guidance for the atmospheric pressure desilication during the Bayer process.
2 Experimental
The DSP precipitation experiments were carried out at 95 °C under different desilication conditions in three necked flasks with agitation made by revolution at 100 r/min. The slurry after desilication was separated by filtration. The filter cakes separated from the slurry samples were washed carefully and dried for weighing and solids tests, while the concentration of filtrate with sodium aluminate solution was determined. The synthetic sodium aluminate solution was prepared by dissolving analytically pure NaAlO2, NaOH, Na2SiO3·9H2O into deionized water. The concentrations of Na2O (ρNa2O) and Al2O3 (ρAl2O3) in sodium aluminate solution before and after the desilication experiments were analyzed by the volumetric method, and the concentration of SiO2 (ρSiO2) was analyzed by spectrophotometry using a 722S spectrophotometer.
X-ray diffraction measurements were performed to identify the minerals in samples with an X-ray powder diffractometer (XRD, PANalytical PW3040/60) using Cu Kα radiation with a scan rate of 3 (°)/min. The total crystallinities of DSPs were calculated using the Jade software. The mass fractions of different crystalline phases in the DSPs were semi-quantitatively calculated by the RIR method. Scanning electron microscopy and energy dispersive X-ray spectroscopy (EDS) were performed on samples using a scanning electron microscope (FESEM, Zeiss UltraPlus), operating at an accelerating voltage of 15 kV.
3 Results and discussion
3.1 Effect of initial SiO2 concentration on DSPs precipitation
The Si-containing minerals in bauxite ore mainly contain kaolinite, illite, quartz, opal, oolitic chlorite, sericite and feldspar, and the reaction activities of which with the alkali solution are quite different. Meanwhile, the contents of active Si-containing minerals in various bauxite ores vary sharply. Therefore, the concentration of silica in sodium aluminate solution during the Bayer process is different. The precipitation behavior of DSPs in synthetic sodium aluminate solution with different initial SiO2 concentrations from 1 to 5 g/L at 95 °C for 8 h was studied.
The concentrations of sodium aluminate solution after desilication are listed in Table 1. The concentrations of caustic alkali, alumina, and silica decrease after the desilication process, especially for the silica concentration dropping by a large percentage. The final silica concentrations with different initial SiO2 concentrations are almost the same, demonstrating that the equilibrium solubility of silica in sodium aluminate solution at 95 °C is about 1.1 g/L. The DSPs concentration formed with different initial SiO2 concentrations was determined after drying, as shown in Fig. 1. No DSP is precipitated when the initial SiO2 concentration is 1 g/L. With the increase of initial SiO2 concentration, the DSPs concentration in sodium aluminate solution increases approximately linearly to the initial SiO2 concentration.
Table 1 Concentration of sodium aluminate solution with different initial SiO2 concentrations before and after desilication
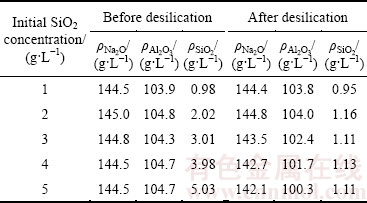
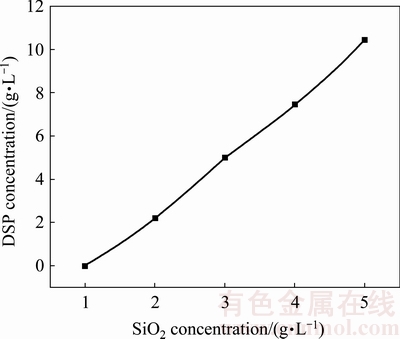
Fig. 1 DSP concentration formed with different initial SiO2 concentrations
The XRD patterns of DSPs precipitated in sodium aluminate solution with different initial SiO2 concentrations are shown in Fig. 2. The effect of initial SiO2 concentration on the mineralogical compositions of DSPs is quite apparent. When the initial SiO2 concentration is between 2 and 3 g/L, the crystalline DSPs comprise ZEO, SOD and ZEO-A. However, the crystalline DSPs only comprise ZEO-A and ZEO when the initial SiO2 concentration is over 3 g/L. The proportions of each DSP among the total crystalline DSPs with different initial SiO2 concentrations were semi-quantitatively calculated, as listed in Table 2, and the corresponding lattice constants were also calculated. With the increase of initial SiO2 concentration, the proportions of ZEO and SOD among the total crystalline DSPs decrease and the corresponding lattice constants increase. In contrast, the proportion of ZEO-A increases largely with the increase of initial SiO2 concentration, and the corresponding lattice constant decreases.
Many researchers reported the existence of amorphous phase of sodium aluminosilicate hydrate formed under the Bayer-type conditions. The total crystallinities of DSPs with different initial SiO2 concentrations obtained from the XRD patterns were calculated, as listed in Table 2. The crystallinity of DSPs increases with the increase of initial SiO2 concentration, demonstrating that increasing the initial SiO2 concentration during the desilication process under atmospheric pressure results in the decrease of the amorphous DSPs.
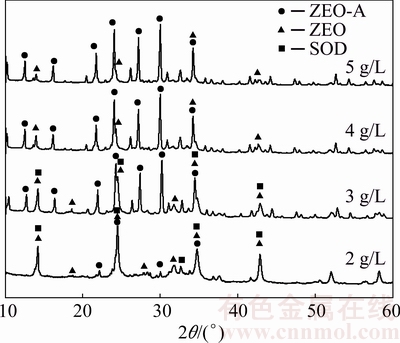
Fig. 2 XRD patterns of DSPs formed with different initial SiO2 concentrations
The microstructures of DSPs precipitated in sodium aluminate solution with different initial SiO2 concentrations were characterized by SEM as shown in Figs. 3 and 4. On a macro level, all the DSPs are precipitated in the forms of agglomerates as seen in Fig. 3(a) and Figs. 4(a), (c), and the agglomeration degree increases with the increasing initial SiO2 concentration. The ZEO appears as quasi-spherical agglomerates with a ‘cotton ball’ type morphology (Fig. 3(b)), and each ‘cotton ball’ contains considerable discs. The irregular-shaped small particles existing between the ZEOs as shown in Fig. 3(c) are the amorphous DSPs. The SOD has a square prismatic particle shape, which either attaches the surface of ZEO or precipitates individually (Fig. 3(d)). The attachment of SOD to the ZEO surface indicates that the SOD is precipitated after the ZEO precipitation.
The ZEO-A with an octahedron shape exists obviously as observed in Figs. 4(b) and (d) when the initial SiO2 concentrations of sodium aluminate solution are 3 and 5 g/L, respectively. The proportion of ZEO-A in Fig. 4(d) is much larger than that in Fig. 4(b), which is consistent with the XRD results as listed in Table 2. As seen in Fig. 4(d), the ZEO is precipitated on the surface of ZEO-A, indicating that the ZEO is precipitated after the ZEO-A precipitation.
Table 2 Proportion and lattice constant (a) of DSPs formed with different initial SiO2 concentrations
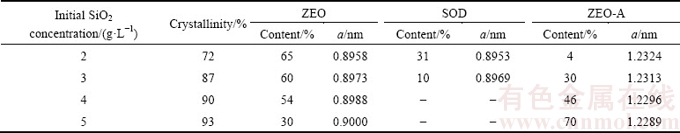
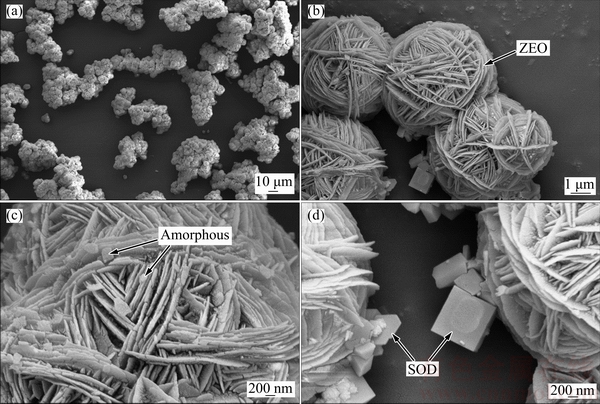
Fig. 3 SEM images of DSPs formed with initial SiO2 concentration of 2 g/L for 8 h
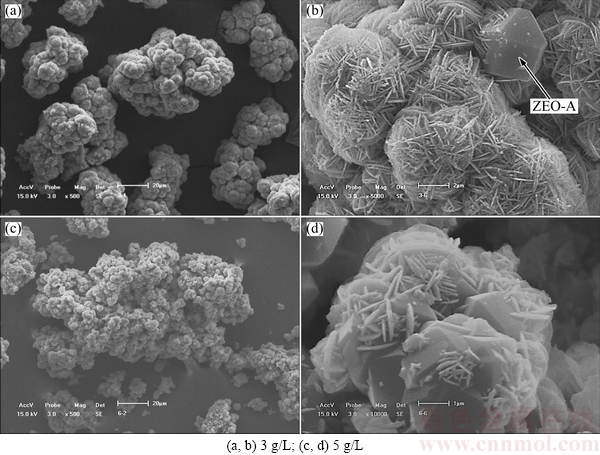
Fig. 4 SEM images of DSPs formed with different initial SiO2 concentrations for 8 h
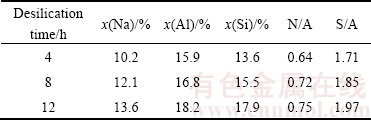
The micro-area compositions of DSPs were determined by EDS, and no obvious difference was found among the different kinds of DSPs precipitated under the same desilication conditions. However, the EDX results of Na, Al and Si in DSPs formed with different initial SiO2 concentrations are different as listed in Table 3, the data of which are the average of at least 3 micro points. The molar ratios of Na2O to Al2O3 (N/A) and SiO2 to Al2O3 (S/A) were calculated accordingly.
Table 3 EDX results of DSPs formed with different initial SiO2 concentrations
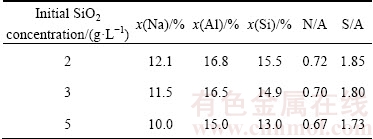
Both the N/A ratio and the S/A ratio of DSPs decrease with the increase of initial SiO2 concentration.
3.2 Effect of duration on DSPs precipitation
The precipitation behavior of DSPs in sodium aluminate solution with different durations at 95 °C when the initial SiO2 concentration is 2 g/L was then studied. The concentrations of sodium aluminate solution after desilication are listed in Table 4. The silica concentration decreases slightly when the desilication duration is less than 2 h, then decreases sharply as the desilication duration increases to 8 h, and finally becomes stable until 12 h. The DSPs concentration formed with different desilication durations are in good agreement with the results of liquor concentrations as shown in Fig. 5. Most of DSPs are precipitated with the duration from 2 to 8 h, and the precipitated DSPs are rare at the start or the end of desilication process.
Table 4 Concentration of sodium aluminate solutions in different durations before and after desilication
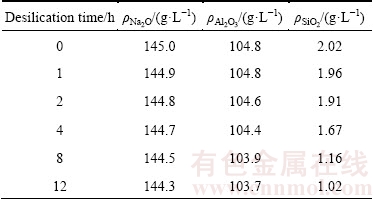
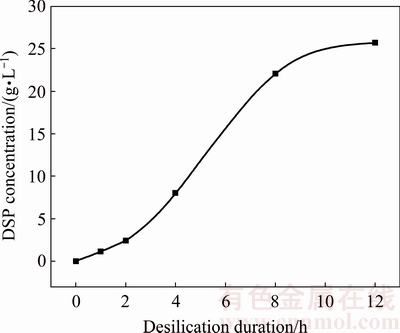
Fig. 5 DSPs concentration formed with different durations
The XRD patterns of DSPs formed with different durations are shown in Fig. 6. The DSPs comprise amorphous phase, ZEO, SOD and ZEO-A when the desilication durations are 4 and 8 h, but the ZEO-A disappears when prolonging to 12 h. As listed in Table 5, the total crystallinity of DSPs increases with the increasing duration, demonstrating that extending the desilication time can decrease the content of amorphous phase. The proportions of each DSP and its lattice constant among the total crystalline DSPs formed with different durations are listed in Table 5, and the corresponding cell volumes of DSPs were calculated as shown in Fig. 7. The proportion of ZEO increases but the proportions of SOD and ZEO-A decrease as the desilication duration increases. All the lattice constants of ZEO, SOD and ZEO-A increase with the increasing duration. As shown in Fig. 7, both the cell volumes of ZEO and SOD increase as the duration increases, and the increase amplitude of ZEO is larger than that of SOD.
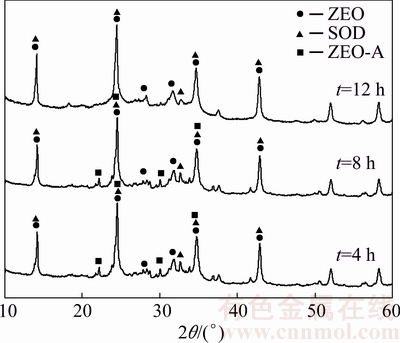
Fig. 6 XRD patterns of DSPs formed with different durations
The EDX results of DSPs formed with different durations are listed in Table 6. Both the N/A ratio and the S/A ratio of DSPs increase with the increasing duration, especially for the S/A ratio. This result is in agreement with the crystallographic data in Table 5 and Fig. 7. The constituent elements of sodium aluminosilicates gradually diffuse into the crystal lattice of DSPs with the increase of reaction time, resulting in the formed DSPs more and more stable.
Table 5 Proportion and lattice constant (a) of DSPs formed with different durations
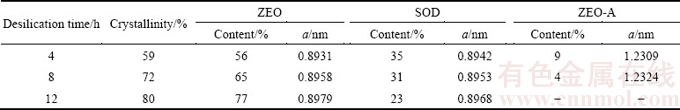
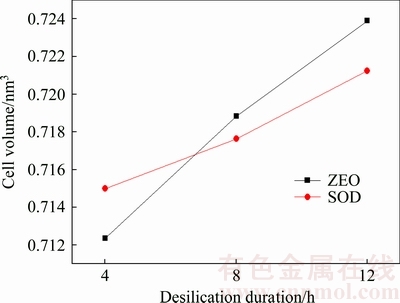
Fig. 7 Cell volume of DSPs formed with different durations
Table 6 EDX results of DSPs formed with different durations
3.3 Effect of initial Al2O3 concentration on DSPs precipitation
The alumina concentrations in sodium aluminate solution before and after the Bayer digestion are quite different. According to the Bayer circle theory, the molar ratio of caustic Na2O to Al2O3 (αK) before digestion is about 3.0, while it decreases to 1.4 after digestion. Therefore, the precipitation behavior of DSPs in sodium aluminate solution with different αK from 1.4 to 3.0 at 95 °C for 8 h when the initial SiO2 concentration is 2 g/L was finally studied. The concentrations of sodium aluminate solution after desilication are listed in Table 7, and the corresponding DSP concentrations formed with different initial αK are shown in Fig. 8. The silica concentration does not change when the αK is 1.4. Because the equilibrium solubility of silica in sodium aluminate solution increases with the increase of alumina concentration, the DSPs are not precipitated when the αK is 1.4 due to the high alumina concentration. The silica concentration after desilication decreases gradually as the αK increases, and the DSP concentration increases accordingly. Increasing the αK of sodium aluminate solution promotes the precipitation of DSPs.
Table 7 Concentration of sodium aluminate solutions with different initial αK before and after desilication
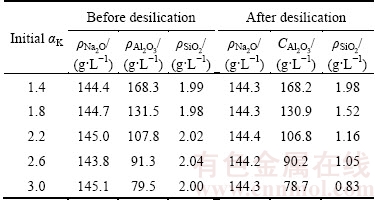
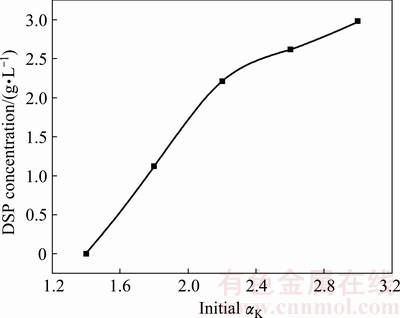
Fig. 8 DSPs concentration formed with different initial αK
The XRD patterns of DSPs with different initial αK are shown in Fig. 9. The DSPs comprise amorphous phase, ZEO and SOD when the initial αK is 1.8. However, when the initial αK increases to 2.2, the ZEO-A starts to precipitate. As listed in Table 8, the increase of initial αK not only promotes the precipitation of ZEO-A, but also decreases the content of amorphous phase and ZEO. On the contrast, the proportion of SOD among the total crystalline DSPs increases largely with the increase of initial αK.
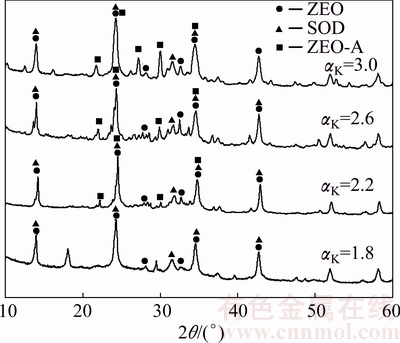
Fig. 9 XRD patterns of DSPs formed with different initial αK
3.4 Discussion
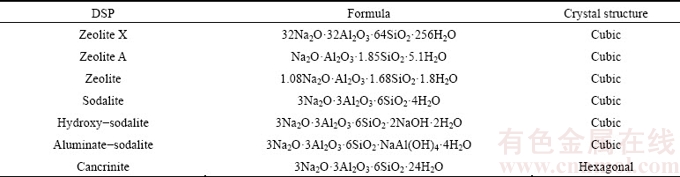
The formation of DSPs in silica-supersaturated sodium aluminate solution is usually represented as Eq. (1). According to the XRD results in this study, the types of DSPs under different desilication conditions are quite different. The molecular formulae and crystal structures of various DSPs without lime incorporation are listed in Table 9 [19]. Most DSPs belong to the cubic crystal except cancrinite. Because of the atmospheric pressure desilication with relatively short duration and synthetic sodium aluminate solution without impurities, the cancrinite was not found under the present desilication conditions. As listed in Table 9, the molecular formulae of DSPs are different. Meanwhile, according to the SEM results in Figs. 3 and 4, the morphologies of DSPs are quite different. Therefore, the various DSPs may not transform each other, and one DSP is precipitated based on the dissolution of another DSP.
xNa2SiO3(aq)+2NaAl(OH)4(aq)→Na2O·Al2O3·xSiO2·(4-x)H2O(s)+2xNaOH(aq) (1)
According to the results in Section 3.2, the amorphous phase and the ZEO-A are unstable DSPs precipitated during the desilication process, the contents of which decrease with the increasing desilication duration. As the duration prolongs, both of the above DSPs dissolve gradually. In contrast, the ZEO and SOD can exist stably during the desilication process. The ZEO is the stablest phase among all the DSPs, the content of which increases with the increasing duration.
According to the results in Section 3.1, the precipitation of ZEO-A occurs at a high silica- supersaturated state in sodium aluminate solution. The larger the driving force of desilication reaction is, the more easily the ZEO-A precipitates. Thus, the content of ZEO-A increases as the initial silica concentration increases. The results of Section 3.3 also prove the above conclusions. The metastable solubility of silica in sodium aluminate solution decreases with the decreasing alumina concentration. As the increase of initial αK, the metastable solubility of silica decreases gradually, and the corresponding supersaturation degree of silica increases [19,20]. Thus, the content of ZEO-A increases as the initial αK increases.
According to the XRD and SEM results in this study, the precipitation sequence of DSPs under atmospheric pressure at 95 °C is: amorphous phase→ ZEO-A → ZEO → SOD. It is consistent with BARNES et al’s results [15], although they did not give the precipitation sequence of ZEO-A because of the different desilication conditions from this study. Most of the previous researches studied the pressurized desilication simulating the bauxite digestion process, and the desilication time was very long even for several days [14,20]. Therefore, the precipitation of ZEO-A was seldom found and studied during the Bayer desilication process. However, the synthesis of zeolite A (Na12Al12Si12O48·27H2O) from various silicon-containing minerals, such as fly ash, kaolin and feldspar, was widely investigated in recent years [21-23]. SU et al [23] described the synthesis mechanism of zeolite A from a pretreated K-feldspar as the following three main stages: 1) the dissolution of amorphous aluminosilicate releasing [SiO2(OH)2]2- and Al(OH)4-; 2) the formation sodium aluminosilicate gel as zeolite precursor; 3) the crystallization of zeolite A. Although the morphology of ZEO-A in this study is different, the forming mechanisms of zeolite A during the desilication processes may be the same.
Table 8 Proportion and lattice constant (a) of DSPs formed with different initial αK
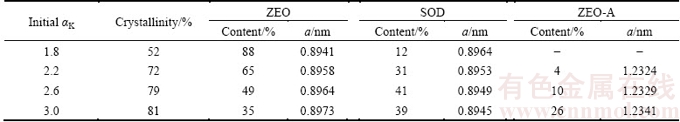
Table 9 Various DSPs formed during desilication process
4 Conclusions
1) The DSPs precipitated in silica-supersaturated sodium aluminate solution at 95 °C comprise amorphous phase, ZEO-A, ZEO and SOD, and the precipitation sequence of DSPs is: amorphous phase → ZEO-A → ZEO → SOD.
2) The DSP concentration and total crystallinity increase with the increase of initial silica concentration, initial αK and desilication duration. Decreasing the initial silica concentration, initial αK and increasing the desilication duration reduce the proportion of ZEO-A.
3) The micro morphologies of various DSPs are quite different precipitated in the forms of agglomerates. The ZEO and SOD are the stable DSPs formed under atmospheric pressure, while the precipitation of ZEO-A occurs at a high silica-supersaturated state in sodium aluminate solution.
References
[1] HIND A R, BHARGAVA S K, GROCOTT S C. The surface chemistry of Bayer process solids: A review [J]. Colloids and Surfaces A—Physicochemical and Engineering Aspects, 1999, 146: 359-374.
[2] SMITH P. The processing of high silica bauxites-review of existing and potential processes [J]. Hydrometallurgy, 2009, 98: 162-176.
[3] YANG Hui-bin, PAN Xiao-lin, YU Hai-yan, TU Gan-feng, SUN Jun-min. Dissolution kinetics and mechanism of gibbsitic bauxite and pure gibbsite in sodium hydroxide solution under atmospheric pressure [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(12): 4151-4159.
[4] ADU-WUSU K, WILCOX W R. Kinetics of silicate reaction with gibbsite [J]. Journal of Colloid & Interface Science, 1991, 143: 127-138.
[5] HO G E, ROBERTSON W A, ROACH G I D, ANTONOVSKY A. Morphological study of Bayer process desilication product and its application to laboratory and plant digests [J]. Industrial & Engineering Chemistry Research, 1992, 31: 982-986.
[6] XU B, SMITH P, WINGATE C, SILVA L D. The effect of calcium and temperature on the transformation of sodalite to cancrinite in Bayer digestion [J]. Hydrometallurgy, 2010, 105: 75–81.
[7] WHITTINGTON B I, FLETCHER B L, TALBOT C. The effect of reaction conditions on the composition of desilication product (DSP) formed under simulated Bayer conditions [J]. Hydrometallurgy, 1998, 49: 1-22.
[8] PAN Xiao-lin, YU Hai-yan, TU Gan-feng. Reduction of alkalinity in bauxite residue during Bayer digestion in high–ferrite diasporic bauxite [J]. Hydrometallurgy, 2015, 151: 98-106.
[9] WHITTINGTON B I, FALLOWS T. Formation of lime–containing desilication product (DSP) in the Bayer process: Factors influencing the laboratory modelling of DSP formation [J]. Hydrometallurgy, 1997, 45: 289-303.
[10] XU B, WINGATE C, SMITH P. Transformation of sodalite to cancrinite under high temperature Bayer digestion conditions [J]. Light Metals, 2009: 51-56.
[11] GASTEIGER H A, FREDERICK W J, STREISEL R C. Solubility of aluminosilicates in alkaline solutions and a thermodynamic equilibrium model [J]. Industrial & Engineering Chemistry Research, 1992, 31: 1183-1190.
[12] GERSON A R, ZHENG K. Bayer process plant scale: Transformation of sodalite to cancrinite [J]. Journal of Crystal Growth, 1997, 171: 209-218.
[13] PARK H, ENGLEZOS P. Thermodynamic modeling of sodium aluminosilicate formation in aqueous alkaline solutions [J]. Industrial & Engineering Chemistry Research, 1999, 38: 4959-4965.
[14] BARNES M C, ADDAI–MENSAH J, GERSON A R. The kinetics of desilication of synthetic spent Bayer liquor and sodalite crystal growth [J]. Colloids and Surfaces A—Physicochemical and Engineering Aspects, 1999, 147: 283-295.
[15] BARNES M C, ADDAI–MENSAH J, GERSON A R. The mechanism of the sodalite–to–cancrinite phase transformation in synthetic spent Bayer liquor [J]. Microporous and Mesoporous Materials, 1999, 31: 287-302.
[16] KAWASHIMA N, SHI L, XU N, LI J, GERSON A R. Characterisation of single-stream Bayer plant heat exchanger scale [J]. Hydrometallurgy, 2016, 159: 75-86.
[17] ARMSTRONG J A, DANN S E. Investigation of zeolite scales formed in the Bayer process [J]. Microporous and Mesoporous Materials, 2000, 41: 89-97.
[18] PAN Xiao-lin, YU Hai-yan, DONG Kai-wei, TU Gan-feng, BI Shi-wen. Pre-desilication and digestion of gibbsitic bauxite with lime in sodium aluminate liquor [J]. International Journal of Minerals, Metallurgy and Materials, 2012, 19: 973-977.
[19] PAN Xiao-lin, YU Hai-yan, TU Gan-feng, BI Shi-wen. Effects of precipitation activity of desilication products (DSPs) on stability of sodium aluminate solution [J]. Hydrometallurgy, 2016, 165: 261-269.
[20] BARNES M C, ADDAI-MENSAH J, GERSON A R. The solubility of sodalite and cancrinite in synthetic spent Bayer liquor [J]. Colloids and Surfaces A—Physicochemical and Engineering Aspects, 1999, 157: 101-116.
[21] OJUMU T V, DU PLESSIS P W, PETRIK L F. Synthesis of zeolite A from coal fly ash using ultrasonic treatment—A replacement for fusion step [J]. Ultrasonics Sonochemistry, 2016, 31: 342–349.
[22] AYELE L,  I. Synthesis of zeolite A from Ethiopian kaolin [J]. Microporous and Mesoporous Materials, 2015, 215: 29-36.
I. Synthesis of zeolite A from Ethiopian kaolin [J]. Microporous and Mesoporous Materials, 2015, 215: 29-36.
[23] SU Shuang-qing, MA Hong-wen, CHUAN Xiu-yun. Hydrothermal synthesis of zeolite A from K-feldspar and its crystallization mechanism [J]. Advanced Powder Technology, 2016, 27: 139-144.
蒋 涛,潘晓林,吴 艳,于海燕,涂赣峰
东北大学 冶金学院,沈阳 110819
摘 要:研究在95 °C下合成过饱和二氧化硅铝酸钠溶液时不同脱硅条件对脱硅产物的生成量、物相组成及其结晶度和显微组织的影响规律。在常压脱硅条件下,脱硅产物由无定型沸石、A型沸石、沸石和方钠石组成;脱硅产物的生成量和结晶度随着初始二氧化硅浓度、分子比和脱硅时间的增加而增加。降低初始二氧化硅浓度和分子比,增加脱硅时间,能够降低A型沸石的含量。沸石和方钠石是稳定的脱硅产物,而A型沸石只在高饱和二氧化硅浓度的铝酸钠溶液中析出。脱硅产物析出后均发生附聚,但不同类型脱硅产物的显微形貌明显不同;脱硅产物中Na2O与Al2O3摩尔比、SiO2与Al2O3摩尔比随着脱硅时间的增加而增大,从而使脱硅产物的晶胞体积增大。常压下过饱和二氧化硅铝酸钠溶液中脱硅产物的析出顺序为:无定型沸石→A型沸石→沸石→方钠石。
关键词:拜耳法;脱硅;铝酸钠溶液;沸石;方钠石
(Edited by Xiang-qun LI)
Foundation item: Projects (51774079, 51674075, 51104041) supported by the National Natural Science Foundation of China; Project (N130402010) supported by the Fundamental Research Funds for the Central Universities, China
Corresponding author: Xiao-lin PAN; Tel: +86-24-83686460; E-mail: panxl@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(18)64670-9

